A brief introduction of purification process of VLPs vaccine
Vaccine
Vaccine belongs to the most effective ways to control and deter the spread of infectious diseases. Pathogens and their metabolites can serve as an immunological formulation to prevent or treat infectious diseases in the manner of artificial attenuation, detoxification, inactivation, or genetic engineering methods[1]. Traditional vaccines including inactivate vaccine and attenuated vaccine can trigger strong and long-term immune responses in cells of host, and therefore plays an indispensable role in the market.
Factors including potential pathogenicity and production disruption dramatically impact the vaccine industry. Innovative vaccines, especially genetic engineering vaccines based on Virus-Like Particles, (VLPs) have successfully gained extensive attention and great popularity due to higher level of immunogenicity and safety(Fig.1) [2].
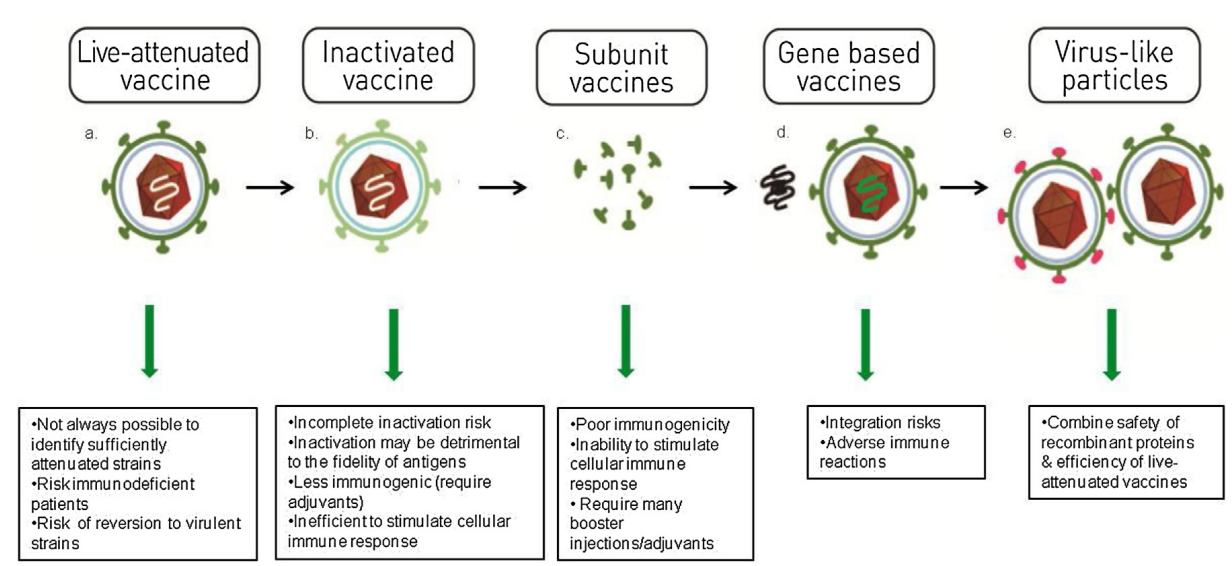
Fig.1 Classification of vaccines
VLPs
VLPs vaccine is based on multi-metric particles which shares similar nanostructures with natural viruses. VLPs composed of one or multiple structural proteins of certain virus, providing them same or extremely similar structure with real viruses. However, the absence of generic materials disables self-replicating of VLPs, leading to its zero risk in pathogenicity or infectivity.
In terms of structure, VLPs consist of enveloped VLP and non-enveloped VLP(Fig.2). VLP vaccines can have double or triple layers of envelopes, achieving polyvalent vaccines and polyvalent vaccine functions. Non-enveloped VLPs, such as HPV,HEV vaccines, enjoy relative simple structure, which can be expressed in prokaryotic or eukaryotic system, especially in the E.Coli and yeast expression system with high expression and easy scale-up.
By contrast, enveloped VLPs possess complex structure, which requires the involvement of host cell membrane, enabling the expression in eukaryotic systems including yeast, insect cells and plant cells[3]. Since the VLP content and ratio can vary in different expression systems, level of immunogenicity can be impacted.
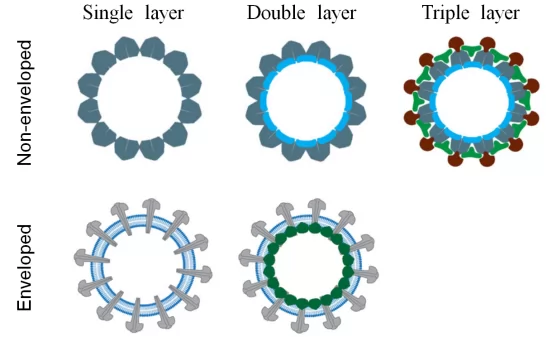
Fig.2 Non-enveloped VLPs and enveloped VLPs
VLPs vaccines available in market
Currently, several VLP based vaccines are available in market(Table1), including Recombivax HB and Engerix-B for hepatitis B virus (HBV), Gardasil, Cervarix and Gardasil-9 for human papilloma virus (HPV) as well as Hecolin for hepatitis E virus(HEV).
Table 1. Available VLP-based vaccines
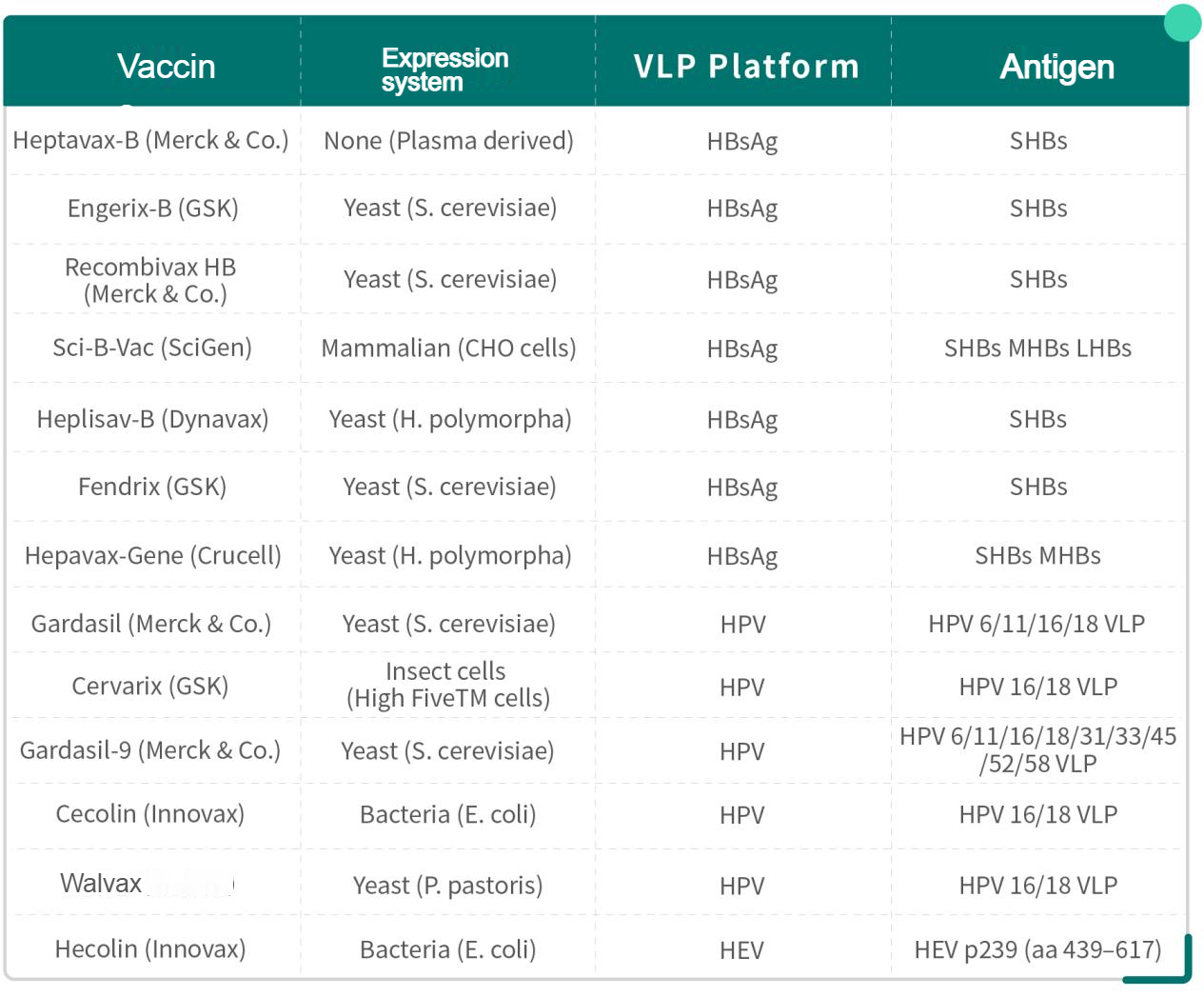
1.HBV vaccine
Hepatitis B virus (HBV) is the major pathogen for Hepatitis B. The virus belongs to the enveloped DNA virus, which has an envelope made of lipid and three types of envelope proteins. Vaccination is by far the most effective way for the prevention of HBV infection[4].
So far, the R&D of LVP based vaccine has reached to the third generation. Heptavax-B is the first generation of vaccine, which is obtained by HBV surface antigen particles via separation of HBV patient blood sample.
Due to the concern in uncertainty of vaccine safety and source, the second generation of vaccine, Recombivax HB(MSD) and Engerix-B(GSK) successfully achieved the approval from government. Based on HBV VLPs genetic engineering, both vaccines use the saccharomyces cerevisiae system to express HBsAg(with a symmetric regular icosahedron structure ).
In 2018, innovative HBV vaccine Heplisav-B got approval and was available for sale. The vaccines is expressed in Hansenyeasts, possessing both recombinant HBsAg and Toll-like receptor 9 agonist adjuvant. It enjoys higher immunogenicity than Engerix-B(which requires one doses every 6 months, 3 doses in total), and only requires 2 shots in total(one month gap between two doses).
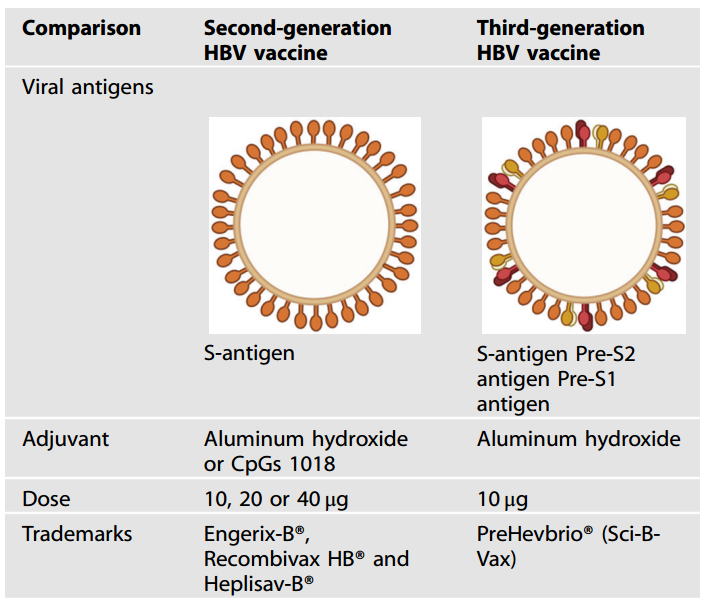
The second generation of vaccine has single antigen(Small S Antigen of HBsAg), which provides long-term serum protection, improving efficacy and protection against HBV. The third generation vaccine Sci-B-Vac(PreHevBrio) expresses three types of HBV surface antigens(S, pre-S1 and pre-S2) in CHO cells, mixing VLPs from glycosylated and non-glycosylated HBsAg and therefore enjoys higher immunogenicity due to its higher resemblance with natural structure[5].
Table.2 Comparison of 2nd and 3rd HBV vaccines
2. HPV vaccine
Cervical cancer is the second most fatal cancer in females worldwide, around 70% cervical cancers are caused by high-risk HPV subtypes 16 and 18. Quadrivalent Gardasil(MSD) is the first approved HPV preventive vaccine. Recombinant protein L1 obtained from yeast expression self-assembles into VLP, providing protection against viral infections caused by HPV 6/11/16/18.
The nine-valent vaccine Gardasil-9 further launched by Merck enjoys higher total antigen number and aluminum adjuvant content. Apart from protection against HPV6/11/16/18, the vaccine offers extra protection against HPV 31/33/45/52/58 infection.
In 2009, FDA approved a bivalent vaccine Cervarix(GSK), which is expressed in baculovirus insect cell expression system, with major targets towards HPV16/18. Due to its innovative AS 04 adjuvant system, the vaccine can provide better immunogenicity and longer protection against HPV16/18.
In December of 2019, Cecolin got the approval from China FDA and was allowed to be used for the prevention of HPV 16/18. Compared with yeast and insect/baculovirus expression systems, Cecolin used E.coli expression system, helping to bring the cost down. Another HPV 16/18 targeted vaccine, Walrinvax, was approved in May,2022 in China. The vaccine was expressed by Pichia pastoris.
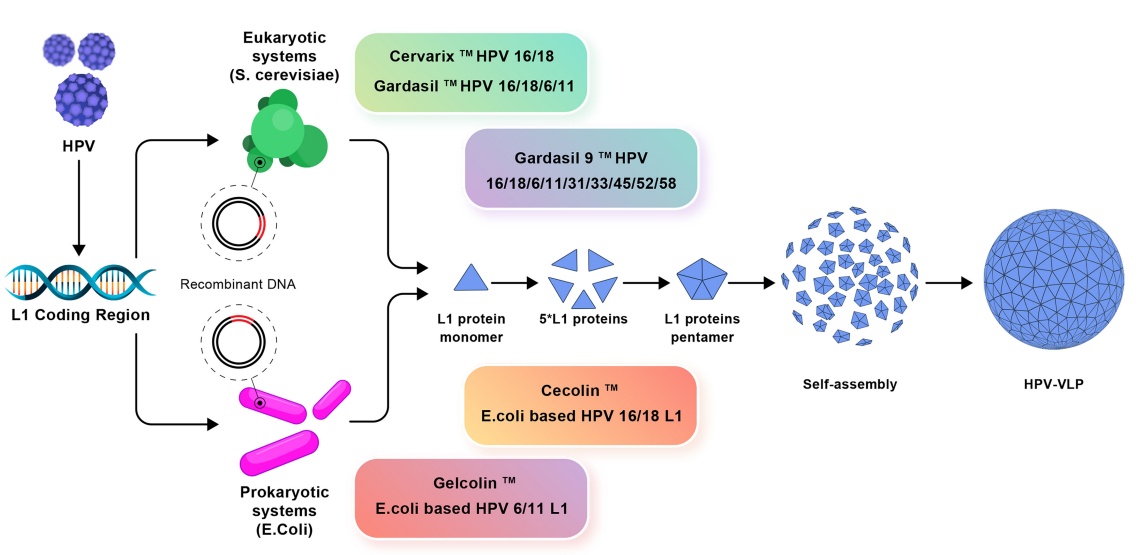
Fig.3 HPV vaccine[6]
By the end of December 31st of 2022, a total of 20 HPV vaccines are in the clinical trial. Among which, nine-valent HPV vaccines from Recbio, WANTAI BioPharm, Walvas Biology, Bovaxbio and Health Guard Biotechnology have entered the Phase Ⅲ clinical trial[7].
HPVs possess two types of proteins for virus coding, specifically L1 and L2. The proteins can form icosahedral capsid and L1 is the major structural protein. Nowadays, the launched preventative HPV vaccines are based on the L1-contatining self-assembled VLPs. Since L1 protein is major structural protein for virus, it is possible to assemble VLPs with high immunogenicity, inducing a strong specific immune response.
Tiny capsid protein L2, the highly reserved protein in HPV, is considered the right antibody target for next generation HPV vaccine R&D.
Schellenbacher et al. constructed a chimeric HPV vaccine (HPV 16 L1-HPV 16 L2) that expresses both L1 and L2 proteins, produces a broad-spectrum neutralizing antibody against HPV 16 under adjuvant induction. [8]
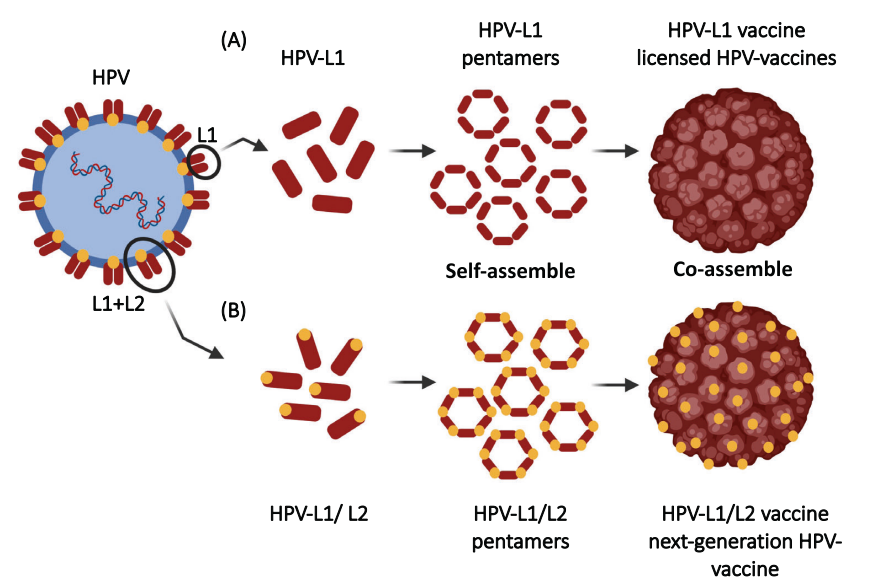
Fig.4 HPV structural protein
3. HEV hepatitis E virus vaccine
HEV is a non-enveloped single-stranded RNA virus whose genome contains three open reading frames, ORF2 encoded unique structural protein. Based on truncated pORF2, HEV239 (Hecolin) by Waitai BioPharm was approved in China in 2012. The vaccine is expressed in E.Coli, becoming the world’s first and the only hepatitis E virus vaccine.
VLP vaccine downstream purification process
The diversity of VLPs expression system prevents the general applicability of downstream purification process. Fig.5 General downstream process flow-chart. [9]
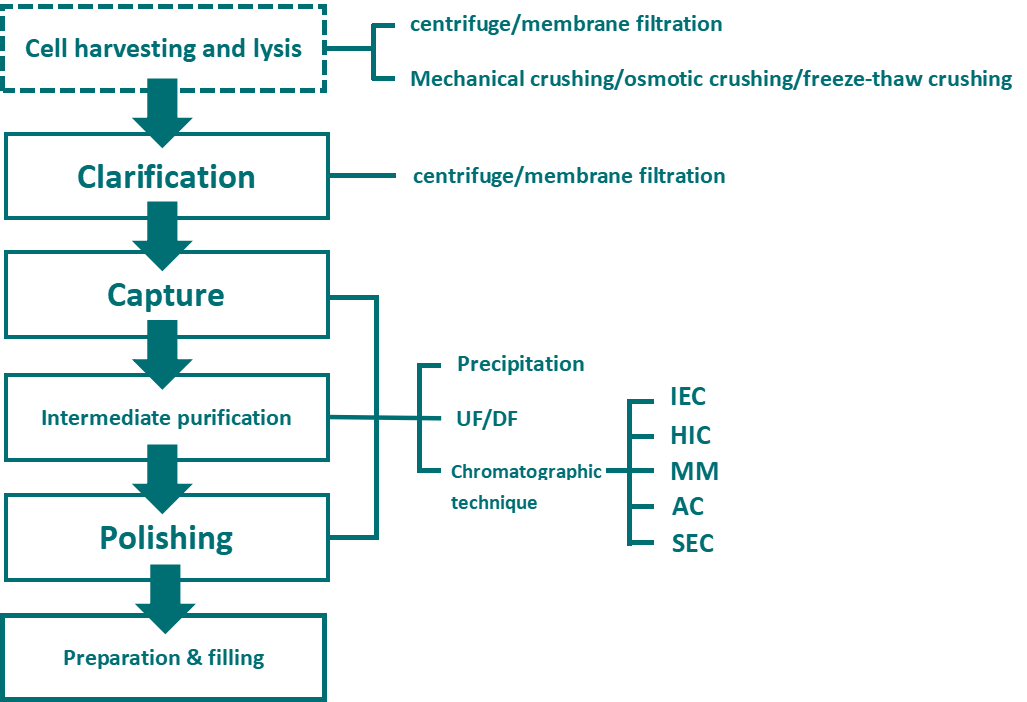
Fig.5 Downstream process for VLP vaccine
Purification include initial purification, intermediate purification and polishing stage. Chromatographic method is the key operation unit in the VLPs downstream process, which includes affinity chromatography, IEX, HIC, SEC and mixed-mode chromatography.
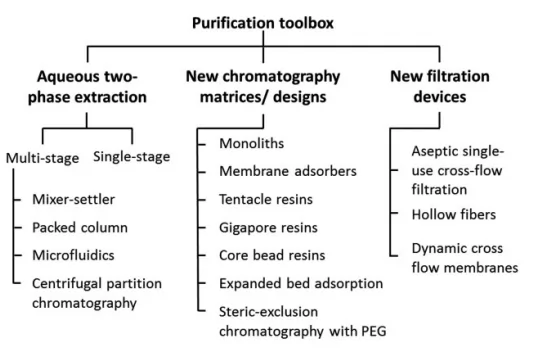
Fig.6 VLPs purification technique
1. HBV vaccine production process
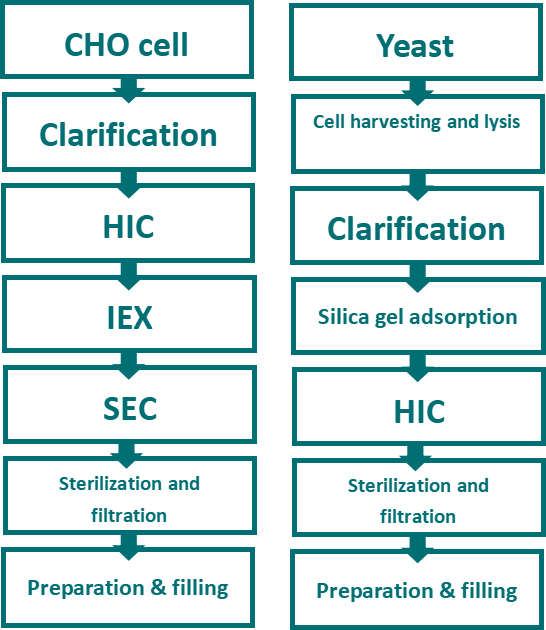
Fig.7 HBsAg preparation process via CHO cells and yeast expression system
Currently, three methods for Hepatitis B vaccine are available in China. Specifically, CHO cell, Hanssen's yeast and brewer’s yeast. Yeast cell usually adopts intracellular expression in the preparation of HBsAg, which shall go through clarification after cell harvesting & lysis. HIC resin is used in the capture stage(hydrophobic bond of HBsAg protein can bind with hydrophobic ligands), AEX resin is used for the removal of HCP under flow-through mode while SEC resin is used for the removal of small molecular weight impurities.
However, CHO cells often use extracellular expression, whose feedstock has small amount of HCP, requiring no lysis procedure. CHO contains glycosylated HBsAg and enjoys more similarity with natural structure. Thus, it is possible to adopt a simpler purification process compared with yeast expression system by choosing resin with weak hydrophobicity in initial capture stage.
Bestchrom provides market with a wide range of HIC resins. Diamond HIC series resins and Diamond HIC Mustang series resins are obtained by coupling HIC functional groups on high rigid agarose beads. The resins enjoy advantages including low back pressure, fast flow, better suitability for maro-biomolecules purification on large scale.

Fig.8 Diamond Butyl resin
2. HPV vaccine production process
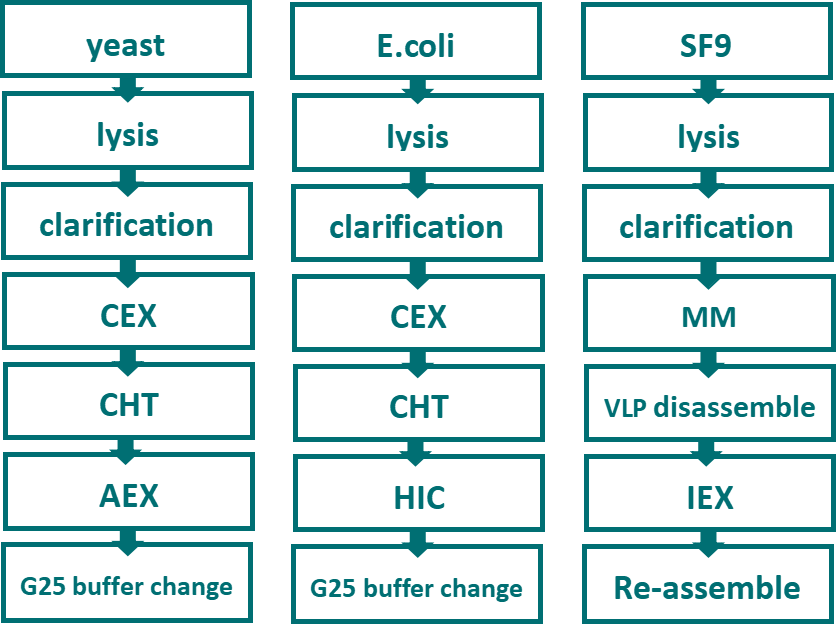
Fig.9 HPV vaccine purification process
Currently, three types of expression systems for available HPV vaccines consist of E.coli, yeast and Baculovirus/insect cells (Fig.9). Downstream process usually adopts AEX resin for capture stage. Bestchrom MegaPoly 50HS enjoys a special pore structure consists of large throughpores and diffusive pores, which can boost binding capacity while mitigating adverse impact of diffusion on chromatographic process, providing merits including high flow, high binding capacity, high resolution as well as a better option for more efficient and rapid purification in capture stage.
The following steps can adopt hydroxypapatite resins(CHT Ⅱ), IEX or HIC resins for polishing. During purification process, it is essential to remove endotoxin for E.coli expression system, whereas the removal of recombinant baculovirus(BV) genome and HCD will be the key for baculovirus/insect cells expression system. IEX resins can effectively remove impurities such as HCP, HCD and colitoxin. Besides, IEX resins with different bead sizes can be used for capture or polishing stages in the chromatographic process.
Bestchrom Diamond IEX resins are obtained by coupling IEX functional groups on high rigid agarose beads with dextran spacer arms, which will boost protein binding capacity and efficiency via improved ligand concentration.

Fig.10 Diamond DEAE (left) and Diamond Q (right) resins
Mixed-mode chromatography plays a dominant role in the VLPs purification R&D due to various interaction forces provided by it.
Bestchrom Diamond Layer 400 and Diamond Layer 700 resins belong to the mixed-mode category, whose molecular weights are 400KD and 700KD respectively. The double-layered structure consists of both core microspheres(having AEX and HIC functions) and thin shell(agarose shell without ligands) with SEC function. Specifically, impurities with smaller molecular weight can enter into microspheres and bind with ligands inside, whereas VLPs will be blocked from microspheres.
Therefore, compared with conventional SEC resins, Bestchrom resins dramatically boost sample loading volume and effectively reduce CV in scale-up. The above two resins are dedicated for the intermediate purification and polishing stages for virus and other macro-biomolecules, providing excellent option for the virus purification in vaccine production.

Fig.11 Diamond Layer 700 resin
To improve quality of vaccine, all expression systems shall go through steps including disassemble and re-assemble. Due to the fact that impurities can be both in the surface and insider of beads, HCP and HCD can be removed more thoroughly and effectively under disassemble status.
Natural HPV containing L1 protein and L2 protein, while HPV vaccines currently available do not contain L2 protein, leading to inconsistency of bead sizes and enhanced immunogenicity. It is possible to get disassemble by adding right amount of DTT in chromatographic process and buffer exchange is needed to remove reductive agent in subsequent re-assemble step.
To get better purification results for VLPs, it is essential to choose the appropriate chromatographic methods according to antibody property and expression system complication in downstream purification. Besides, it is recommended to combine membrane chromatography technique to improve immunogenicity and product safety.
Reference
[1] Recent Progress on the Versatility of Virus-Like Particles. VACCINE, 2020, 8, 139.
[2] Production of virus-like particles for vaccines. NEW BIOTECHNOLOGY, 39 , pp.174-180.
[3] Bioengineering Virus-Like Particles as Vaccines. BIOTECHNOLOGY AND BIOENGINEERING,111 (3) , pp.425-440.
[4] Hepatitis B vaccine development and implementation. HUMAN VACCINES &IMMUNOTHERAPEUTICS, 16 (7) , pp.1533-1544.
[5] Virus-like particle vaccinology, from bench to bedside. CELLULAR & MOLECULAR IMMUNOLOGY ,19 (9) , pp.993-1011
[6] An Update on Human Papilloma Virus Vaccines History Types Protection and Efficacy. FRONTIERS IN IMMUNOLOGY,12
[7] Research report on current situation and future trend of human vaccine market
[8] Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. JOURNAL OF VIROLOGY, 83 (19) , pp.10085-10095.
[9] Next generation vaccines and vectors: Designing downstream processes for recombinant protein-based virus-like particles. BIOTECHNOLOGY JOURNAL, 10 (5) , pp.715-U100.








.png)


