Downstream Purification Strategies for Antibody–Drug Conjugates
Antibody–drug conjugates (ADCs) are often described as “biological missiles” in cancer therapy. By leveraging the high specificity of monoclonal antibodies to target tumor cells, ADCs enable the precise delivery of cytotoxic payloads, significantly enhancing therapeutic efficacy while reducing systemic toxicity. Since the approval of the first ADC drug, Mylotarg, in 2000, more than 17 ADC drugs have been approved worldwide as of 2024, spanning both hematological malignancies and solid tumors, with a global market value exceeding USD 10 billion. However, the structural complexity of ADCs imposes stringent requirements on manufacturing processes. This article provides an in-depth analysis of downstream purification strategies for ADCs, the associated technical challenges, and corresponding solutions.
Overview of Quality Attributes of Antibody–Drug Conjugates
The core objective of bioprocess development is to establish a robust and well-controlled manufacturing process that ensures the reproducible production of products meeting predefined quality attributes.
For traditional non-ADC biologics, critical quality attributes (CQAs) include key parameters that may impact clinical safety and efficacy, such as product size-related variants (e.g., aggregates and fragments), charge heterogeneity, and oxidative variants, as well as process-related impurities (e.g., host cell proteins and residual DNA), product concentration, and biological activity.
In contrast, due to the dual nature of ADCs as both biological macromolecules and small-molecule drugs, additional quality attributes must be carefully considered, including the drug-to-antibody ratio (DAR), the distribution of DAR species, residual free payload, and residual solvents from the conjugation reaction.
• DAR value and DAR species distribution: These parameters directly impact the efficacy and safety of ADC drugs. Manufacturing processes must ensure lot-to-lot consistency of both the DAR value and its distribution.
• Free payload: As a high-risk process-related impurity, unconjugated small-molecule payloads lack a targeted delivery mechanism and therefore cannot selectively act on tumor cells. Instead of contributing to therapeutic efficacy, they may pose a risk of systemic toxicity.
• Residual solvents from the conjugation reaction: During process development, attention must also be paid to the removal efficiency of process-related impurities introduced during the conjugation reaction, including organic solvents (e.g., DMF, DMSO) and reducing agents (e.g., TCEP), to ensure that the final product meets predefined quality release criteria.
Downstream Purification of Antibody–Drug Conjugates
◉ Removal of Free Payload by Ultrafiltration/ Diafiltration (UF/DF)
Following the conjugation reaction, free payload and residual solvents are typically present and must be removed. Ultrafiltration/diafiltration (UF/DF) is a commonly used method for buffer exchange in biopharmaceutical processing. By selecting UF/DF membranes with an appropriate molecular weight cutoff, low-molecular-weight impurities—including residual solvents and free payload—can be efficiently removed while retaining the product. As shown in Fig. 1, under ideal conditions UF/DF can reduce post-conjugation impurities to acceptable levels.
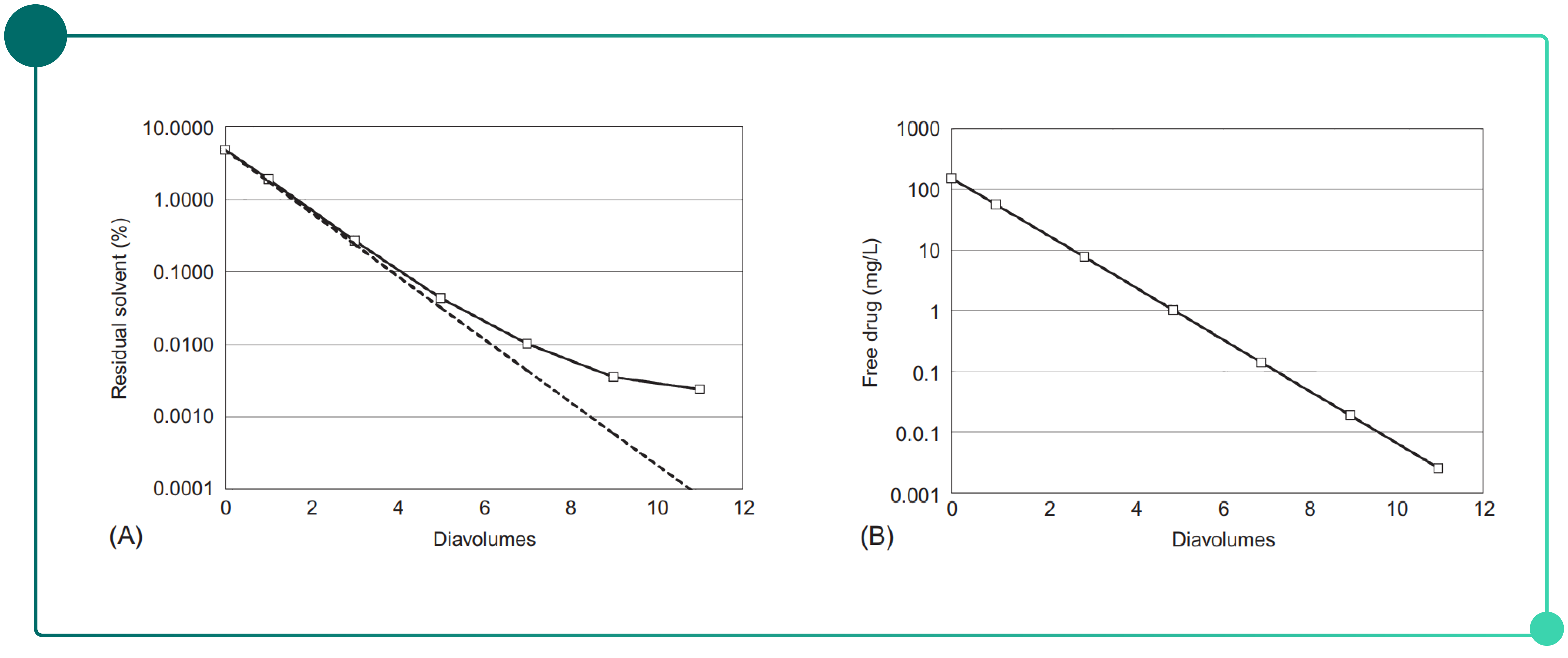
Fig. 1. Impurity clearance during UF/DF as a function of diafiltration volume.
(A) Residual solvents (B) Free payload
For certain ADCs, free payload and payload-related impurities may not be effectively removed by UF/DF. As shown in Fig. 2, during UF/DF performed using a 30 kDa membrane, the clearance behavior of two free drug–related impurities, both with molecular weights below 2 kDa, was evaluated. Impurity A was able to pass through the UF/DF membrane and was removed by more than 2 log during this step, whereas impurity B was not significantly cleared even after 10 diavolumes of diafiltration. This behavior may be partially attributed to self-association of the payload, which increases its apparent size in aqueous environments, or to nonspecific interactions between the free payload and the ADC.
• If UF/DF fails to reduce free payload and related impurities to acceptable levels, chromatographic purification steps are required.
• If the conjugation reaction results in unacceptable levels of product variants or aggregates, chromatographic purification of the ADC is necessary.
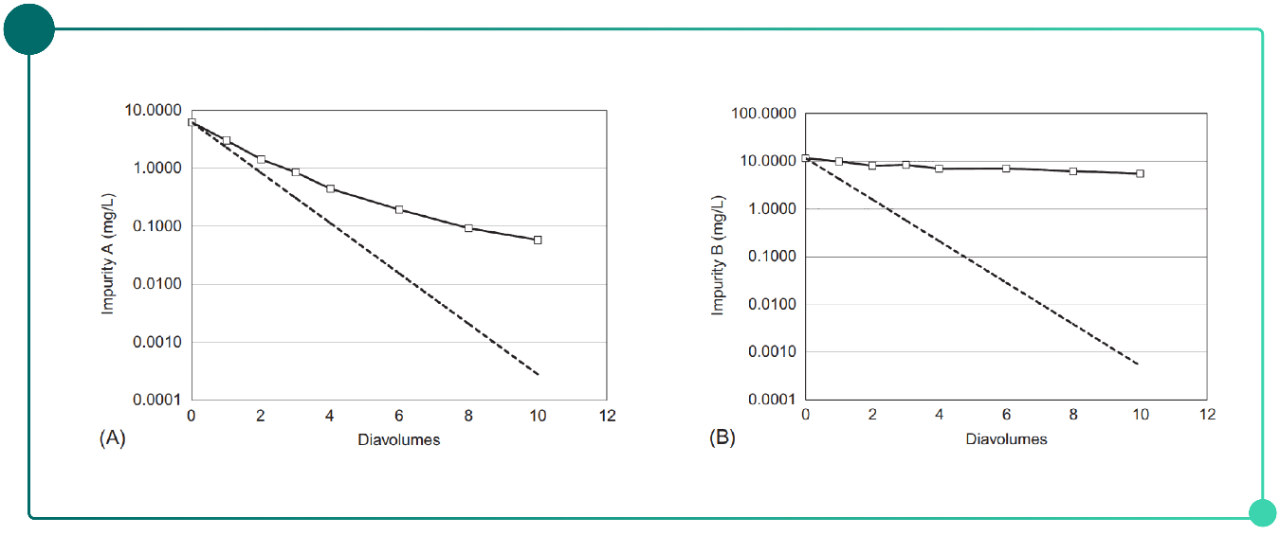
Fig. 2. Impurity clearance during UF/DF as a function of diafiltration volume.
(A) Residual solvents (B) Free payload
◉ Removal of Free Payload by Cation Exchange Chromatography
Bind-and-elute chromatography, as a conventional separation technique, can be effectively applied to remove free payload from ADCs. This approach exploits the pronounced differences in physicochemical properties between ADCs and free payload molecules, enabling efficient separation through selective adsorption.
In cation exchange chromatography, ADCs—whose unconjugated antibodies typically exhibit relatively high isoelectric points—carry a net positive charge under low-conductivity, acidic loading conditions and are therefore strongly retained on the resin. In contrast, most free payload molecules are small in molecular size and are neutral or negatively charged, preventing their binding to the resin and allowing them to be directly removed in the flow-through. As shown in Fig. 3, the free payload content can be significantly reduced from 52.2% to 5.0% through flow-through collection during sample loading and subsequent washing with equilibration buffer.

Fig. 3. Cation exchange chromatography purification profile
Challenges and Optimization
Challenge:
Highly hydrophobic payloads may exhibit nonspecific adsorption to the resin or to the ADC, resulting in incomplete clearance.
Solutions:
Screening cation exchange resins with different ligands;
Adding low concentrations of organic solvents or surfactants to the wash buffer.
◉Removal of Aggregates by Cation Exchange Chromatography
Due to the complexity of ADC manufacturing processes, aggregate levels may increase to unacceptable levels following the conjugation step. As a result, the introduction of a post-conjugation purification step may be necessary to ensure that the process meets the required product quality attributes. In this context, bind-and-elute cation exchange chromatography can be applied to effectively remove aggregates from ADCs. As shown in Fig. 4, this approach enables efficient separation based on the physicochemical differences between aggregates and monomers.
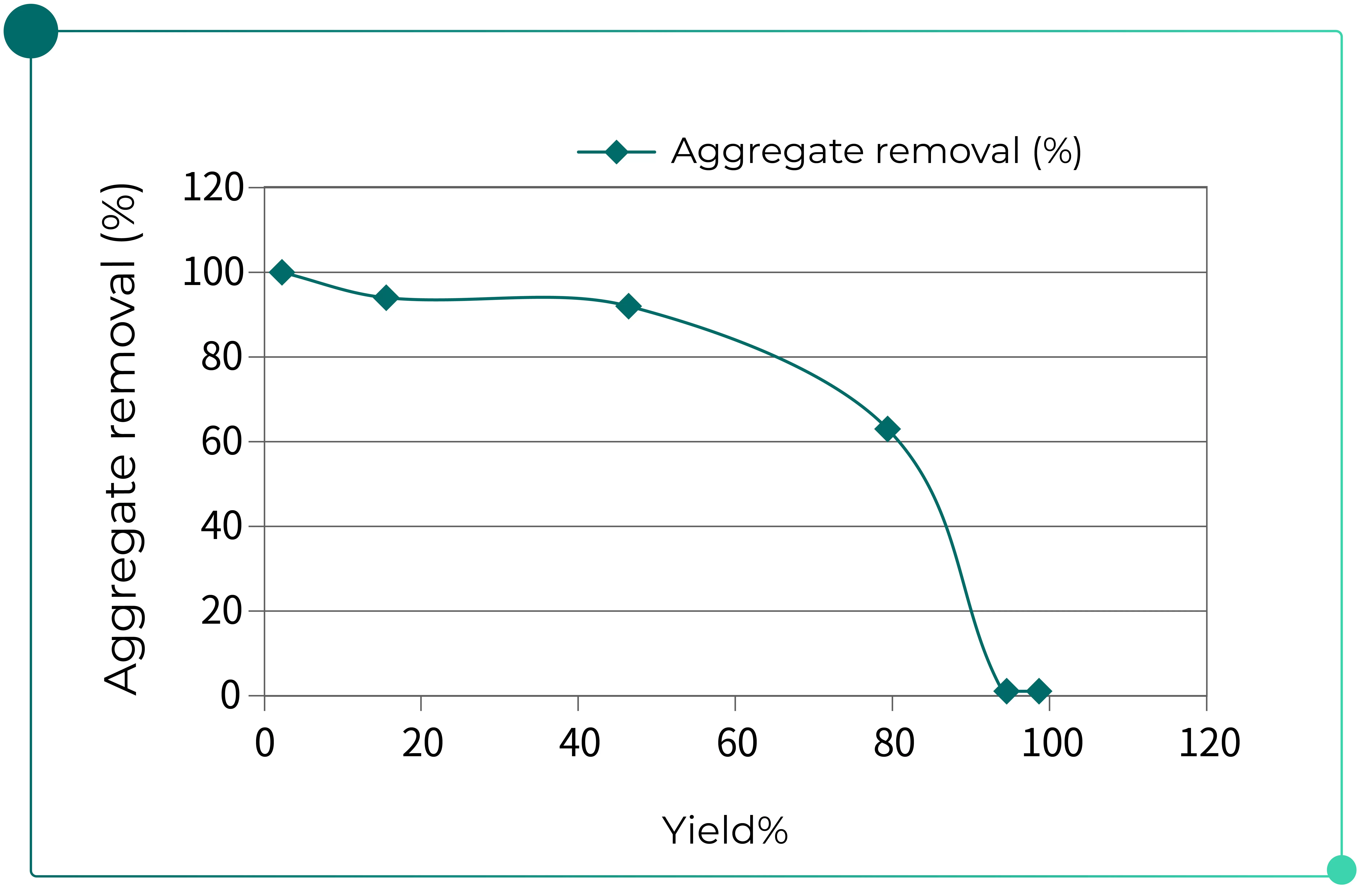
Fig. 4. Aggregate removal by cation exchange chromatography
Control of DAR by Hydrophobic Interaction Chromatography
Because ADCs with different DAR values exhibit different hydrophobicities, hydrophobic interaction chromatography (HIC) can be used to control the DAR profile. As shown in Fig. 5, DAR 0 species have low hydrophobicity and are typically not retained on the HIC resin, passing through during sample loading. In contrast, ADCs with DAR 1 and DAR 2 are captured by HIC and are sequentially eluted during the gradient elution.
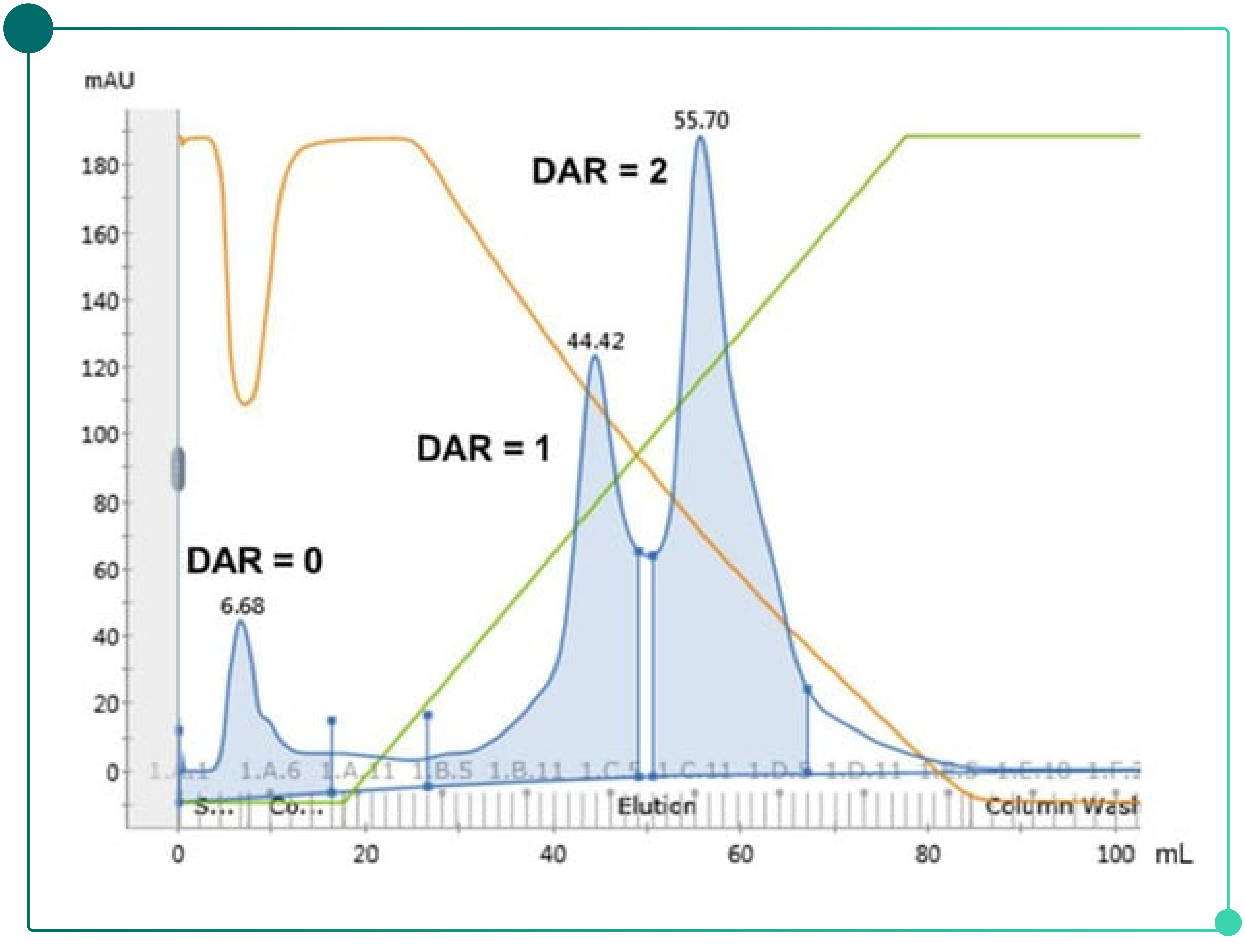
Fig. 5. Separation of ADCs with different DAR values by hydrophobic interaction chromatography
Conclusion
Due to the dual nature of ADCs as both biological macromolecules and small molecules, their purification requires not only the removal of conventional impurities, but also the clearance of free payload, residual solvents from the conjugation reaction, and control of the DAR value and DAR species distribution.
To achieve these objectives, multiple downstream purification strategies are required:
• UF/DF enables rapid buffer exchange and removes the majority of low-molecular-weight impurities.
• Cation exchange chromatography, such as Diamond CD-S—which offers high binding capacity and good tolerance to organic solvents—leverages charge differences to achieve effective removal of free payload as well as efficient clearance of aggregates.
• Hydrophobic interaction chromatography (HIC) exploits differences in hydrophobicity among ADC molecules with different DAR values to enable fine separation, thereby allowing precise control of the DAR value and DAR species distribution.
References
[1] Dumontet, Charles , J. M. Reichert , and L. A. Beck . "Antibody-drug conjugates come of age in oncology." Nature reviews Drug discovery 22.8(2023):641-661.
[2] Hutchinson, Matt H., et al. "Process development and manufacturing of antibody-drug conjugates." Biopharmaceutical processing. Elsevier, 2018. 813-836.
[3] Hendricks, Rachel , et al. "Simplified strategy for developing purification processes for antibody-drug conjugates using cation-exchange chromatography in flow-through mode." Journal of chromatography, A: Including electrophoresis and other separation methods 1666-(2022):1666.
[4] Liu, Huifang et al. A cation exchange chromatography purification method for antibody–drug conjugates. CN104208719A, 2014.
[5]Matsuda Y, Leung M, Okuzumi T, Mendelsohn B. A Purification Strategy Utilizing Hydrophobic Interaction Chromatography to Obtain Homogeneous Species from a Site-Specific Antibody Drug Conjugate Produced by AJICAP™ First Generation. Antibodies (Basel). 2020 May 18;9(2):16. doi: 10.3390/antib9020016. PMID: 32443479; PMCID: PMC7344391.









.png)


