Application of cation exchange chromatography in the purification of ADC
Known as "biological missiles" for tumor treatment, Antibody-drug conjugates (ADCs) uses antibodies to target tumor cells and accurately release cytotoxic loads, which greatly improves efficacy while reducing systemic toxicity.
Since the birth of first ADC drug Mylotarg in 2000, more than 17 ADCs have been approved(by the end of 2025), covering the treatment of Hematoma and solid tumor with an estimated market value of more than 10 billion US dollars. However, its nature of complicated structure(which is composed of antibody,linker and payload as illustrated in Fig.1 ) sets an extreme high bar for production processing. This article will focus its discussion on cation exchange (CEX)chromatography and its application in the efficient purification of ADC drugs.
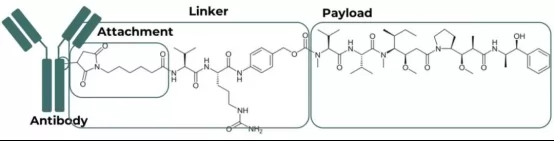
Fig.1 Structure of Anti-CD30 MMAE conjugate (brentuximab vedotin; Adcetris®)
A Brief introduction of the quality requirements of ADC drugs
The key target for biologics production process is to build a stable and controllable production system as well as, the reproducibility of quality-complied products. For traditional Non-ADC biologics, its Critical Quality Attribute(CQA) will cover key parameters which can possibly impact clinical safety and efficacy of drugs, including product size relevant variant(e.g. aggregate,antibody fragments),charge isomers and oxidized variants, as well as process-related impurities(e.g. HCP,residual DNA),concentration and activity.
Since ADC enjoys compatibility to bio-macromolecules and chemical small molecules, it is essential to consider other quality related factors including Drug-to-Antibody Ratio(DAR), composition of DAR , residual free toxins and residual coupling reaction solvents.
Free toxin belongs to high risk process-related impurities. Due to the lack of targeted delivery mechanism, such unconjugated small molecules will not specifically act on therapeutic targets and fail to improve efficacy, which may lead to systemic toxicity risk. In addition, process development should also consider factors including the introduction of organic solvents (e.g.DMF,DMSO)to conjugation reaction system, and clearance efficiency of process-related impurities such as reducing agents(e.g.TCEP), so as to ensure that the final product meets the quality attribute release requirements.
The application of AEX in ADC purification
The removal of free toxins
As a common separation method, bind/elute method can effectively remove the free toxins from ADC drugs. This method achieves the efficient separation via selective adsorption based on the significant disparity in chemical and physical properties.
During chromatography process, first adjust the conjugation reaction system (which contains free toxin and solvents)to targeted sample loading condition. Then load sample to chromatography column, when ionic interaction can selectively capture ADC whereas let free drugs and residual solvents to flow through due to the unavailability of effective binding sites.
However, some chromatographic resins can non-specifically interact with non-target components(e.g. free toxin)in addition to the non-specific interaction between free toxins and ADC. Therefore, it is recommended to systematically select resins, and introduce strengthened washing step to ensure the complete removal of free toxin if necessary.
Fig.2 shows a typical procedure of free toxin removal by using bind/elute mode of CEX resin. Effective removal of free toxins(from 52.2% to 5%)can be achieved by continues buffer washing during sample load and equilibration, enabling the effective removal of free toxin from ADC.
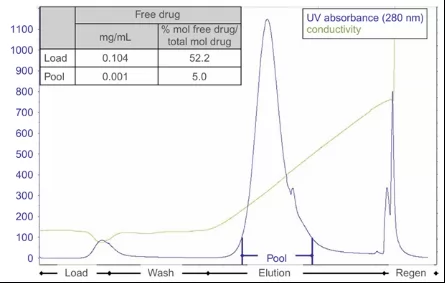
Fig.2 Chromatogram of CEX resin.
Table 1. Purification method using CEX resins

Aggregate removal by using CEX resin
The complexity of ADC production process leads to the rise of protein aggregate content to unacceptable level after conjugation process. Therefore, purification step is necessary after conjugation to ensure the quality of product.
The bind/elute method of CEX can achieve the removal of protein aggregates in ADC. As illustrated, this method enables efficient separation based on the difference of chemical and physical properties between protein aggregates and monomers. ADC conjugation is performed after reduction reaction of disulfide bonds between chains. Refer to the following description for purification method:
• Buffer A: 90mM NaAC-HAC,pH5;
• Buffer B: 400mM NaAC-HAC,pH5;
• Binding capacity: 60mg/mL;
• Elution: 0→100% of Buffer B,20CV of linear gradient elution,Collect fractions in a segmented format.
The following graph shows an example of effective protein aggregate removal from ADC by using CEX resin.
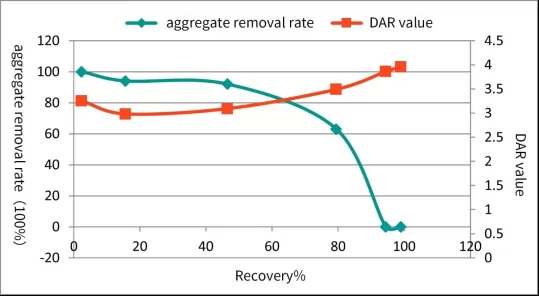
Fig.3 Aggregate removal by using CEX resin
CEX resin provided by Bestchrom
Diamond CD-S CEX resin provided by Bestchrom can be used for the polishing of mAb,BsAb and fusion protein, meeting the requirements of free toxin and aggregate removal in ADC production process. The resin enjoys the following advantages:
• High dynamic binding capacity. The resin enjoys high DBC under high salinity(with 10% DBC can reach up to 100g/L,see Fig.4).
• High rigid beads of agarose, enjoy advantages such as high flow, low back pressure and max pressure at 5bar, making it suitable for production-scale application.
• Excellent selectivity, good batch-to-batch consistency.
• Good tolerance towards organic solvents(resin enjoys good stability after 7-day equivalent exposure to 15% DMSO at 40 ℃), suitable for the purification of ADC drugs.
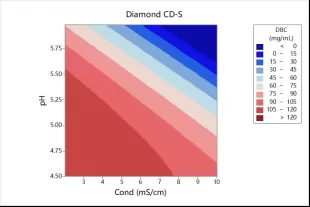
Fig.4 DBC of Diamond CD-S
In addition, Bestchrom offers other CEX resin such as Diamond SP Mustang to cover the whole purification process from antibody polishing to ADC purification.
Order Information
|
Resin |
Pack size |
Cat.No |
|
Diamond CD-S |
25mL |
AI05501 |
|
Diamond SP Mustang |
25mL |
AI0191 |








.png)


