Applications of Chromatography in the In Vitro Diagnostics (IVD) Field
In Vitro Diagnostics (IVD) refers to analytical testing performed on blood, body fluids, or tissue samples outside the human body. It is a core technology for clinical diagnosis and health management, often described as the “eyes of clinicians.”
In recent years, driven by growing demand across disease screening, infection monitoring, immunoassays, and molecular diagnostics, In Vitro Diagnostics (IVD) technology is advancing rapidly. From ELISA and chemiluminescence immunoassays (CLIA) to point-of-care test (POCT), high-quality raw proteins and high-performance separation materials have become the core foundation of diagnostic products.
Chromatography, with its high selectivity, scalability, and mild operating conditions, has become a core technology for producing raw materials used in IVD applications.
Industry Value Chain and Importance of Raw Materials
The IVD value chain generally consists of three segments: upstream raw material supply, midstream reagent and instrument manufacturing, and downstream end-user applications.
◉ Upstream: Raw Material Supply (the Core Segment)
This stage includes biological components such as antibodies, antigens, and diagnostic enzymes, as well as key materials like magnetic beads and carrier materials.
The purity, activity, and lot-to-lot consistency of these materials directly determine the performance of IVD reagents, making this the segment with the highest technical barriers in the entire value chain.
◉ Midstream: Reagent and Instrument Manufacturing
This stage covers the R&D, production, and quality control of a wide range of diagnostic reagents—including immunoassay, clinical chemistry, and molecular diagnostics—as well as the associated analytical instruments.
As global market competition intensifies and cost pressures continue to rise, midstream manufacturers are placing increasing emphasis on the cost efficiency and supply stability of upstream raw materials.
◉ Downstream: End-User Applications
The downstream segment includes hospitals, independent clinical laboratories, public health and disease-control systems, POCT sites, and home-testing scenarios. These end users are highly sensitive to the stability and lot-to-lot consistency of diagnostic reagents.
Why Do IVD Raw Materials Rely Heavily on Chromatography?
Unlike the biopharmaceutical industry, the IVD sector places greater emphasis on
detection specificity and signal stability when evaluating raw proteins. For example:
◉ Requirements for Antigen/Antibody Proteins
• High purity (typically >95%) to minimize background signals
• Free of aggregates or fragments to ensure uniform coating
• Intact conformation and correct epitopes to guarantee detection specificity
◉ Requirements for Enzymes (e.g., HRP, ALP)
• High and stable enzymatic activity
• Free of metal-ion inhibitors
• No small-molecule impurities that may interfere with substrate reactions
◉ Requirements for Ligands on Carrier Materials
• Stable binding capacity
• High lot-to-lot consistency
• Strong chemical stability
Higher purity, greater stability, and stronger consistency in raw proteins form the foundation of high-quality IVD products. Chromatography remains the most robust and reliable technological pathway for achieving these critical attributes.
Typical Applications of Chromatography in IVD Raw Material Preparation
◉ Antibody Purification: Protein A/G/L Affinity Chromatography Followed by Polishing Steps
Antibodies are the most widely used raw materials in IVD, including capture antibodies, detection antibodies, conjugated antibodies, secondary antibodies, and control antibodies. The typical purification workflow is as follows (Fig.1):

Fig. 1 Typical Workflow for Antibody Raw Material Purification
◉ Purification of Enzymatic Proteins (e.g., HRP, ALP, G6PD): Ion Exchange Chromatography
IVD applications place extremely high demands on enzyme activity; therefore, the purification workflow must avoid elevated temperatures and harsh acidic or alkaline conditions. A commonly used chromatography combination includes:
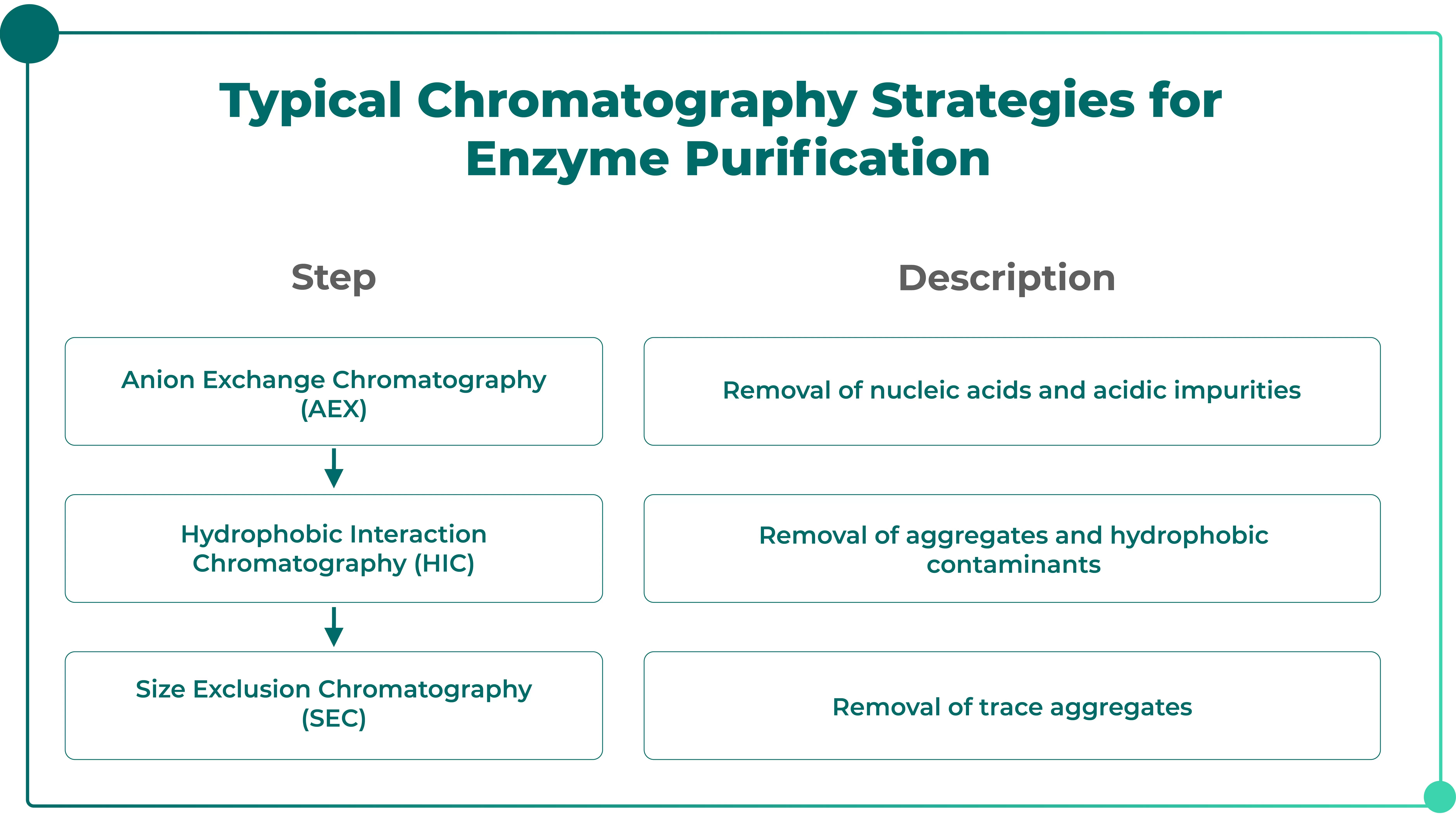
Applicable to:
• Chemiluminescent HRP raw materials
• Dry-chemistry enzyme reagents
• Enzymes used in hematology diagnostics
For recombinant proteins carrying affinity tags, tag-specific resins can be used, such as:
• His-tag → Ni²⁺-NTA
• GST-tag → Glutathione affinity
This approach enables one-step high-purity isolation under non-denaturing conditions, helping maintain the protein’s native conformation.
◉ Preparation of Antigens: Mixed-mode Chromatography
In the IVD field, many critical antigens are produced in recombinant form. Recombinant expression offers a stable, safe, and scalable source of raw materials, while ensuring epitope integrity and lot-to-lot consistency—both essential for immunodiagnostic applications. Common recombinant antigens include:
• Viral antigens: HIV, HBsAg, HCV, etc.
• Tumor markers: AFP, CEA, etc.
• Autoimmune-related antigens
These recombinant proteins often exhibit complex conformations and diverse impurity profiles. Therefore, antigen purification often relies on ion exchange, hydrophobic interaction, or mixed-mode chromatography, as well as activated resins or metal-chelate chromatography.
Mixed-mode chromatography combines mechanisms such as SEC/IEX, HIC, and H-bond interactions, significantly enhancing separation selectivity and impurity removal efficiency.
◉ Surface Ligand Preparation for Diagnostic Magnetic Beads and Affinity Resins
Commonly used materials in IVD include:
• Affinity magnetic beads (Protein A/G/L, Ni²⁺, antibody-coupled beads)
• Solid-phase support materials (e.g., ELISA coating materials)
Chromatography can be applied for:
• Preparation of affinity ligands
• Removal of small-molecule impurities prior to coupling
• Fractionation and purification of ligands based on activity
Application Cases
◉ Case 1: Mouse IgG Purification
• Resin used: AT Protein A Diamond

Fig. 3. SDS-PAGE Analysis of Purified Mouse IgG
Lane 1: Marker; Lane 2: Elution, Batch 1; Lane 3: Elution, Batch 2
Lane 4: Elution, Batch 3; Lane 5: IgG from competitor; Lane 6: Elution, Batch 4
◉ Case 2: Purification of His-Tagged Enzymes
• Resin used: Ni Bestarose FF

Fig. 4. SDS-PAGE Results of Sample Fractions
Left panel: Lane 1: Whole cell lysate; Lane 2: Supernatant; Lane 3: Pellet; Lane 4: Flow-through; Lane 5: Wash; Lane 6: Marker
Right panel: Lanes 1–11: Collected elution fractions; Lane 12: Marker
Chromatography: The Core Technology for Ensuring Stability of IVD Raw Materials
From antibodies and antigens to recombinant enzymes, the purity, structural integrity, impurity profile, and lot-to-lot consistency of IVD raw materials directly determine the performance of diagnostic kits.
Among all available preparation methods, chromatography—with its inherent advantages of:
• High selectivity
• Mild conditions
• Strong impurity-removal capability
• Ease of scale-up
• Excellent process controllability
serves as the primary technology for IVD raw material purification.
“Precise separation empowers diagnostics.” Bestchrom is committed to advancing high-quality development in the IVD industry together with our partners.








.png)


