From R&D to application: GLP-1 purification strategy
Application of GLP-1 drugs
GLP-1 drugs are gaining great attention in recent years for its significant efficacy in diabetes treatment and weight control. In addition to diabetes and obesity, GLP-1 drugs have been widely applied in clinical trials in various diseases, including fatty liver disease, cardiovascular disease, Alzheimer's disease, Parkinson's disease, and chronic kidney disease.
What is GLP-1?
Glucagon-like peptide-1 (GLP-1)is a physiological peptide hormones secreted by intestinal L cells based on glucagon gene editing. Due to biological activity,GLP-1(7-37)will be released several minutes after glucose or food intake, having blood glucose concentration-dependent hypoglycemic effect.
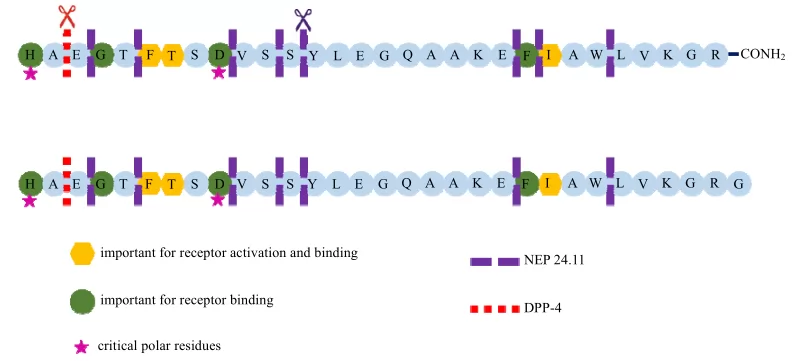
Fig.1 GLP-1 structure diagram
GLP-1 drugs on market
Current GLP-1 drugs available on market include benaglutide, exenatide, liraglutide, lixisenatide, PLGA-encapsulated long-acting exenatide, albiglutide, dulaglutide, Semaglutide, polyethylene glycol loxenatide injection, and oral semaglutide.
Table 1 currently available GLP-1 drugs
|
GLP-1 Drugs |
Company |
Disease |
Frequency |
First approved time |
|
Byetta(exenatide) |
AstraZeneca |
Type 2 diabetes |
Twice a day |
2005(US) |
|
Victoza(liraglutide) |
Novo Nordisk |
Type 2 diabetes |
Once a day |
2009(EU) |
|
Bydureon(exenatide microspheres) |
AstraZeneca |
Type 2 diabetes |
Once a week |
2011(EU) |
|
Lyxumia(Lixisenatide) |
Sanofia Aventis |
Type 2 diabetes |
Once a day |
2013(EU) |
|
Tanzeum(albiglutide) |
GSK |
Type 2 diabetes |
Once a week |
2014(EU) |
|
Saxenda(liraglutide) |
Novo Nordisk |
Obesity |
Once a day |
2014(US) |
|
Trulicity |
Eli Lilly |
Type 2 diabetes |
Once a week |
2014(US |
|
Benaglutide |
Shanghai Benemae |
Type 2 diabetes |
Three times a day |
2016(CN) |
|
Ozempic(semaglutide) |
Novo Nordisk |
Type 2 diabetes |
Once a week |
2017(US) |
|
PEG-Loxenatide |
Hansoh |
Type 2 diabetes |
Once a week |
2019(CN) |
|
Rybelsus(semaglutide) |
Novo Nordisk |
Type 2 diabetes |
Oral medicine |
2019(US) |
|
Wegovy(semaglutide) |
Novo Nordisk |
Obesity |
Once a week |
2021(US) |
|
Mounjaro(tirzepatide) |
Eli Lilly |
Type 2 diabetes |
Once a week |
2022(US) |
GLP-1 related peptide hormone
Glucose-dependent insulin stimulating polypeptide(GLP), Glucagon-like peptide-1(GLP-1) and Glucagon(GCG)are peptide hormones to stabilize glucose level. Their cognate receptors GIPR, GLP-1R and GCGR belong to type B1 G protein-coupled receptor (GPCR) family. Multi-targets or single-molecule peptides with combined agonistic effects of GIPR, GLP-1R and GCGR have been widely explored.
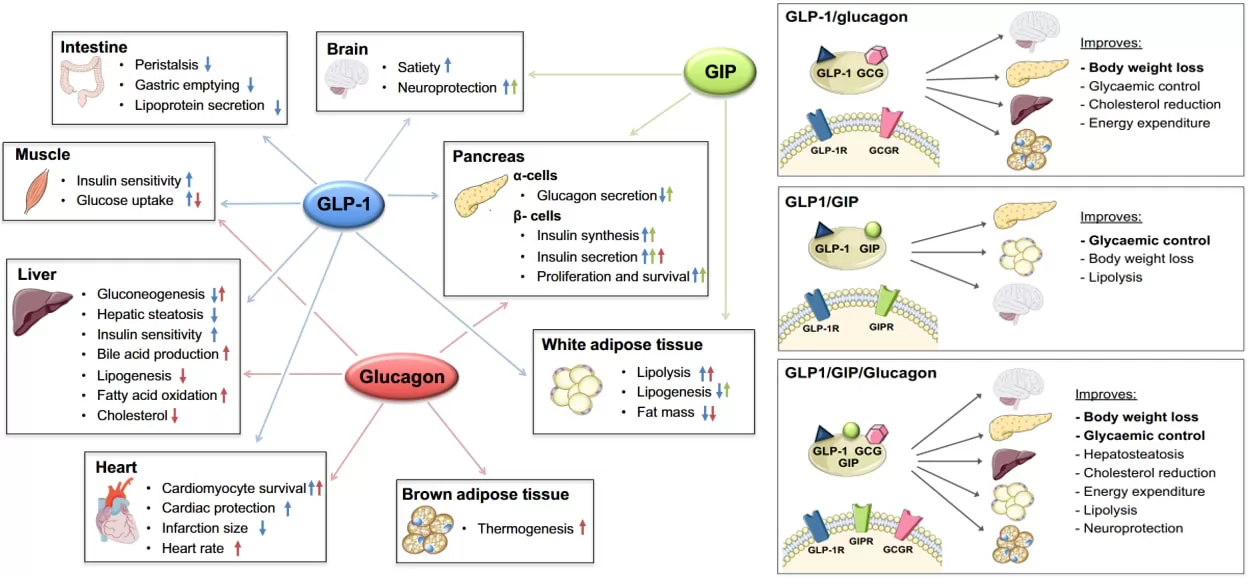
Fig.2 co-metabolic reaction of GLP-1, GIP and GCG
Long-term strategy
Long-term transformation strategies for GLP-1 analogue are:
1) Amino acid sequence optimization: Reduce the rapid degradation of DPP-4 enzyme,extend the half-life.
2) Fusion protein: Use fusion partners such as albumin and Fc to increase the molecular weight of the drug, reduce rapid filtration by the kidneys, coordinate with other action mechanisms to increase the circulation time in the body.
3) Fatty acid modification: On the one hand, the interaction between fatty acids and albumin utilizes the in vivo metabolism and circulation of albumin; on the other hand, the circulation half-life is extended through the self-polymerization and ionicity of fatty acids.
4) PEGylation modification: Increase the size of drug molecules and slow down enzyme degradation.
5) Polymer sustained-release agents: Develop sustained-release dosage forms to extend drug release time.
_1755853267_WNo_1149d742.webp)
Fig.3 Long-term modification characteristics of launched drugs.
GLP-1 production process
Production process of GLP-1 drugs consists of biological fermentation and chemical synthesis.
Currently, majority launched peptide based drugs are prepared via chemical synthesis (solid phase and liquid phase synthesis), with solid phase synthesis the primary method. The amino acid sequence of GLP-1 analogues is less than 50. Non-natural amino acids are introduced through solid-phase synthesis for precise control at fixed points. The production cycle is short and the crude product yield is high. Subsequently, through reverse-phase purification technology, GLP-1 analogues that meet the purity requirements can be obtained.
_1755853304_WNo_587d407.webp)
Fig.4 Principle diagram of solid phase peptide synthesis (SPPS)
Based on the molecular design disparity, upstream expression platforms for biology fermentation includes E.coli expression, yeast expression system and CHO cell expression system. The following are purification process for three types of known molecules:
GLP-1 analogues fusion protein purification process
_1755853368_WNo_1000d350.webp)
Fig.5 Flow chart for GLP-1 analogues fusion protein purification process
GLP-1 analogues Fc protein purification process
_1755853406_WNo_1000d350.webp)
Fig.6 Flow chart for GLP-1 analogs Fc protein purification process
Fatty acids acylate GLP-1 purification process
_1755853449_WNo_1000d350.webp)
Fig.7 Flow chart for fatty acids acylate GLP-1 purification process
Case study
The application of BestPoly 15RPC in peptide purification
Molecular weight of target peptide: 3.4KD
Resin: BestPoly 15RPC
Column size: 1.0*15(cm)-11.8mL
|
Step |
Buffer |
Flow (mL/min) |
|
Equilibration |
0.1% TFA, 10% acetonitrile |
1 |
|
Sample loading |
A: 0.1% TFA, 10% acetonitrile |
0.5 |
|
Elution |
B: 0.1% TFA, 90% acetonitrile |
0.5 |
|
Column preservation |
0.1% TFA, 90% acetonitrile |
1 |
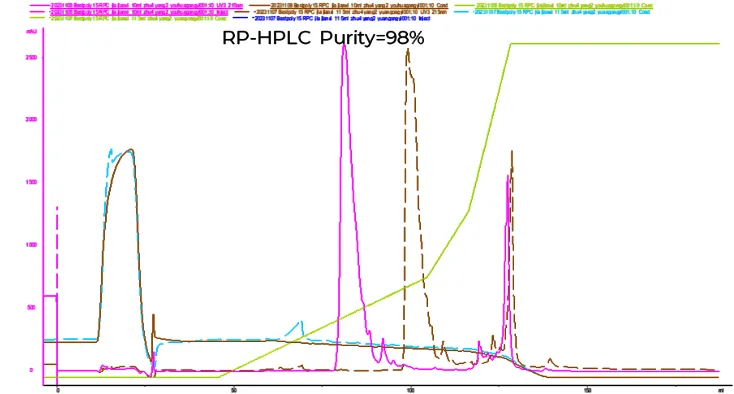
Fig.8 Experiment condition and chromatogram
Order information
|
Resin |
Type |
Pack size |
Cat.No |
Function |
|
Blue Bestarose FF |
Affinity |
25mL |
AA210305 |
Capture fusion protein in albumin |
|
AT Protein A Diamond Plus |
Affinity |
25mL |
AA402305 |
Capture Fc-containing protein |
|
Diamond Q |
AEX |
25mL |
AI0111 |
High rigid matrix, bind target protein via AEX resin property |
|
Q Bestarose FF |
AEX |
25mL |
A10021 |
Capture protein via AEX resin, usually for initial capture stage. |
|
Diamond Butyl |
HIC |
25mL |
AH0111 |
High rigid matrix, capture protein via HIC, high purity |
|
Butyl Bestarose 4FF |
HIC |
25mL |
AH0011 |
Capture protein via HIC, high purity , can also be used for the purification of intermediate products before modification |
|
Diamond S |
CEX |
25mL |
AI0181 |
Capture proteins via CEX |
|
SP Bestarose FF |
CEX |
25mL |
A10011 |
Capture proteins via CEX |
|
Bestpoly 15RPC |
RPC |
25mL |
AR400005 |
Medium pressure chromatography of polypeptides, purification of intermediate products |
Visit www.bestchrom.com for more product information on different pack sizes.
Order products via the following methods:
Call: 400-820-5172
Email: info@bestchrom.com
Reference
[1] Jiang Jingqing, Fu Ling, TAN Menglu, Chen Shengfu. Research progress of glucagon-like peptide-1 (GLP-1) analogues [J]. Chinese Journal of Chemical Engineering, 2019,34(06):1327-1338.
[2] S J Brandt, T D Müller, R D DiMarchi, M H Tschöp, K Stemmer Peptide-based multi-agonists: a new paradigm in metabolic pharmacology J Intern Med . 2018 Dec;284(6):581-602
[3] T. Vilsboll, H. Agerso, T. Krarup, J.J. Holst, Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects, J. Clin. Endocrinol.Metab. 88 (2003) 220–224.
[4] C. Chaudhury, S. Mehnaz, J.M. Robinson, W.L. Hayton, D.K. Pearl, D.C. Roopenian,C.L. Anderson, The major histocompatibility complex-related Fc receptor for IgG(FcRn) binds albumin and prolongs its lifespan, J. Exp. Med. 197 (2003) 315–322.
[5] S. Tzaban, R.H. Massol, E. Yen, W. Hamman, S.R. Frank, L.A. Lapierre, S.H. Hansen,J.R. Goldenring, R.S. Blumberg, W.I. Lencer, The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity, J.Cell Biol. 185 (2009) 673–684
[6] S. Tzaban, R.H. Massol, E. Yen, W. Hamman, S.R. Frank, L.A. Lapierre, S.H. Hansen,J.R. Goldenring, R.S. Blumberg, W.I. Lencer, The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity, J.Cell Biol. 185 (2009) 673–684
[7] Wang, L., Wang, N., Zhang, W. et al. Therapeutic peptides: current applications and future directions. Sig Transduct Target Ther 7, 48 (2022).
[8] Otvos Jr, L., & Wade, J. D. (2023). Big peptide drugs in a small molecule world. Frontiers in Chemistry, 11








.png)


