From source to production process: the control and removal of endotoxin
Introduction: the threat and importance of endotoxin
Endotoxin, an indispensable structural component of the outer membrane of gram-negative bacteria, is a toxic substance released during bacterial reproduction, disintegration and autolysis. It is composed of Lipopolysaccharide (LPS).
In the production of biologics, endotoxin is a common source of contamination posing serve negative impact. Specifically, in addition to compromising purity of biologics, endotoxin can trigger strong immune response, inducing adverse reactions such as fever and shock in patients under severe circumstances. Therefore, the detection and removal of endotoxin is crucial in the production process of biologics, safeguarding the safety of finished products.
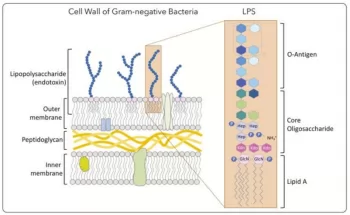
Fig.1 Structure of endotoxin(Montiro & Faciola,2020)
Endotoxins exist in the forms of monomers, micelles or cryptomere. Endotoxins gather in the form of micelles, cubes, layers, or vesicles. It appears as net negative charge in drug solutions. Specifically, negatively charged endotoxin micelles bind on polycationic ligands, alternatively, individual endotoxin monomers can be removed by interaction of hydrophobic lipid tail and hydrophobic surface.
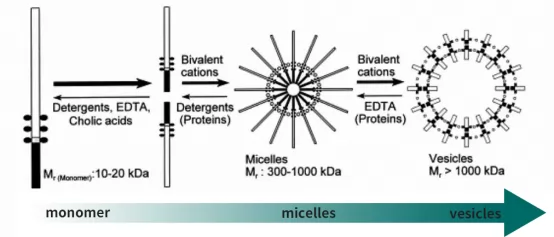
Fig.2 Existed forms of endotoxin
Main source of endotoxin contamination
Two major sources of endotoxin contamination
1.Production system: In genetic engineering, E.Coli.is used for the expression of recombinant protein. During the breakage of cell wall and purification process, large quantity of endotoxin will be released to solutions and become major pollutant. For example,10% wet bacteria can usually produce a volume of endotoxins as large as tens of thousands of EU/mL.
2. Raw material and environmental contamination: even adopting non-endotoxin-producing expression systems such as CHO cell or yeast, it is still possible to introduce endotoxin due to contaminants from raw materials, solutions, production environment and operations.
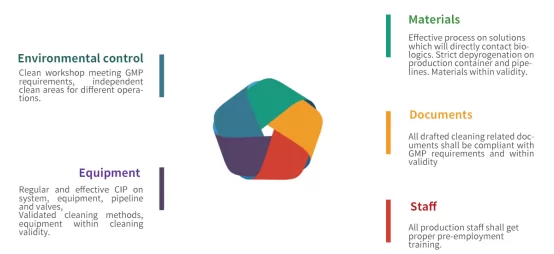
Fig.3 Endotoxin contamination control in production process.
Detection method of endotoxin
To safeguard the safety of biologics, endotoxin detection is a step which can never be skipped. Commonly used detection methods include:
● The rabbit pyrogen test(RPT): RPT, as the earliest pyrogen detection method, is still recorded in the current Chinese Pharmacopoeia. Rabbit has similar response to pyrogens as people,intravenous injection at auricular margin is often used to measure rabbit body temperature, so as to detect pyrogen.This method is very conventional, but has certain limitations in sensitivity and environmental-friendliness.
● LAL (Limulus Amebocyte Lysate) method: This includes both gel method and photometric method. The gel method consists of gel limit test and gel semi-quantitative test, which are the “arbitration” methods specified by the Chinese Pharmacopoeia The photometric method is further divided into turbidity method (including endpoint turbidity method and kinetic turbidity method) and chromogenic substrate method (including endpoint chromogenic method and kinetic chromogenic method). This method utilizes the gel enzyme in the LAL to react with endotoxins and is widely used for detection. It offers advantages such as simplicity of operation and high sensitivity.
● Recombinant Factor C method: using recombinant Factor C as reagent, which can both effective detect endotoxin and reduce overdependence on natural resources(horseshoe crab).It is environmental friendly and persistent.
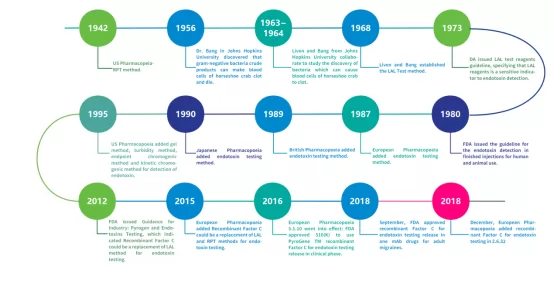
Fig.4 The development of endotoxin detection
Chromatography process in endotoxin removal
Effective removal of endotoxin is the key for safety and quality of biologics. A good understanding of endotoxin structure is the base for its effective clearance. Currently available chromatography methods for endotoxin removal include HIC, IEX , affinity chromatography and SEC.
1.Anion exchange (AEX)chromatography: endotoxin carries negative charge when pH>2,therefore can be bound and removed by positively-charged resin.AEX resin is suitable for samples at low salinity. Under FT mode, keep the sample pH under the pI of target protein and let sample positively-charged. In this way, resin can bind endotoxin while letting target molecules flow through.
2. HIC: HIC method uses high salinity to enhance the bind between hydrophobic protein and resin.When salinity is high, endotoxin will aggregate due to high hydrophobicity of lipid A, which prevents its binding with resin and eventually lead to the effective removal of endotoxin.
3. SEC: endotoxin can form into 1000kDa aggregates via nonpolar and ionic interaction in aqueous solutions, which makes its molecular weight significantly different from most bio-molecules. Based on this feature, SEC resin can effectively isolate endotoxin molecules from target proteins.
4. Affinity chromatography: Effective removal of endotoxin from sample can be achieved through ligands which can specifically adsorb endotoxins(e.g. Polymyxin B).
5. Mixed-mode chromatography: by combining the charge, hydrophobic and hydrogen bond interactions, mixed-mode resin can effectively remove endotoxins by one-step operation, suitable for projects requiring high purity.
The detection and clearance of endotoxins core step to ensure the safety and quality of biologics. The right choice of technology and resin can significantly reduce endotoxin content and improve purification efficiency, so as to meet strict regulatory compliance globally. When facing the challenge of endotoxin removal, Bestchrom provides a wide range of resin solutions for customers in pharmaceutical sectors to solve problems by improving product quality and efficiency.
Product Information
|
Resin |
Type |
Pack size |
Cat.No. |
|
Q Bestarose FF |
AEX |
25mL |
AI0021 |
|
DEAE Bestarose FF |
AEX |
25mL |
AI0031 |
|
Phenyl Bestarose FF |
HIC |
25mL |
AH0031 |
|
Octyl Bestarose 4FF |
HIC |
25mL |
AH0051 |
|
Butyl Bestarose 4FF |
HIC |
25mL |
AH0011 |
|
Diamond MIX-A |
Mixed-mode |
25mL |
AI0101 |








.png)


