Overview of Antibody Affinity Chromatography: A Systematic Guide from Binding Principles to Resin Selection
Antibodies: Structure and Purification Challenges
Antibodies are specific immunoglobulins (Ig) produced by the body in response to antigen stimulation and serve as key effector molecules in humoral immune responses. Each antibody molecule consists of two heavy chains (H) and two light chains (L) (Fig. 1). Based on the amino acid composition and arrangement of the heavy-chain constant region, antibodies are classified into five types: IgM, IgD, IgG, IgA, and IgE — among which IgG is the most common form used in therapeutic antibodies.
Within the same immunoglobulin class, differences in the hinge-region amino acid composition and the number and position of heavy-chain disulfide bonds give rise to subclasses. For example, human IgG is subdivided into four subclasses: IgG1, IgG2, IgG3, and IgG4. Light chains occur in two forms—kappa (κ) and lambda (λ)—thus antibodies are further categorized into κ-type and λ-type immunoglobulins.
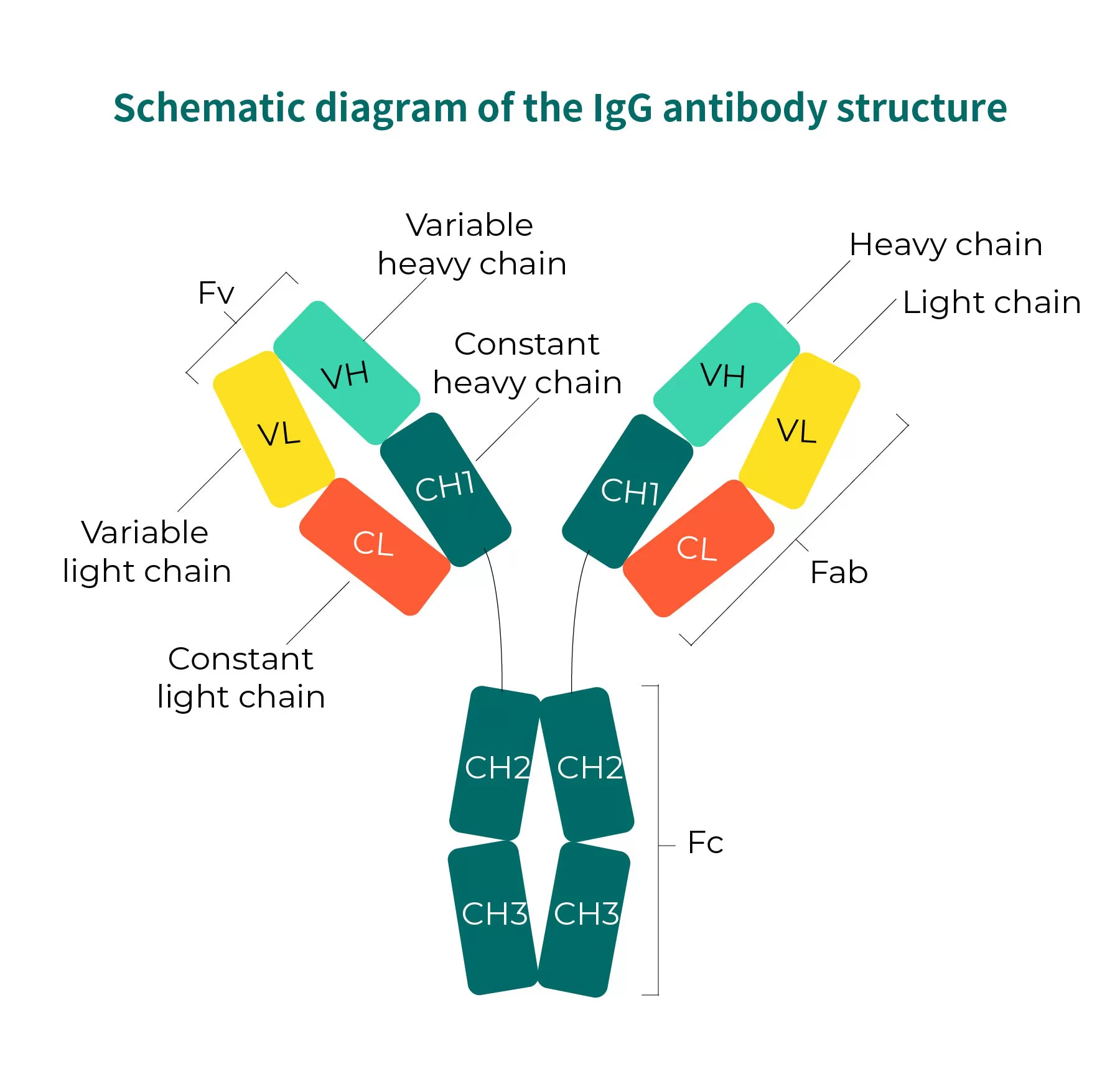
Fig. 1 Schematic diagram of the IgG antibody structure.
With the continuous advancement of antibody drug technology, antibody types and molecular structures have become increasingly diverse.
New molecular formats continue to emerge, ranging from traditional monoclonal antibodies (mAbs) to bispecific antibodies (BsAbs), antibody–drug conjugates (ADCs), antibody–oligonucleotide conjugates (AOCs), and multivalent antibodies.
These molecules differ significantly in Fc structure, binding affinity, glycosylation pattern and thermal stability, posing new challenges for downstream purification processes:
· Increasing variation in Protein A binding:certain antibodies or fragments fail to bind stably.
· Increased molecular sensitivity: low-pH elution may trigger conformational changes or aggregation.
· Impurities increasingly similar to the target molecule: fragments, aggregates, and mis-paired molecules exhibit charge and hydrophobicity similar to the main peak, making separation more difficult.
· Narrower operation window: higher demands are placed on ligand selection, elution conditions, and washing strategies.
Therefore, developing affinity-chromatography processes that combine high selectivity with mild elution conditions has become a key direction for optimizing antibody purification workflows.
Principles of Antibody Affinity Chromatography
Antibody affinity chromatography is a classical method that leverages specific biomolecular interactions to achieve highly selective separations.
The most commonly used ligand is Protein A—a membrane protein derived from Staphylococcus aureus that binds specifically to the Fc region of antibodies, enabling one-step capture with high purity.
Native Protein A contains five IgG-binding domains but also exhibits non-specific interactions; modern resins therefore employ engineered Protein A variants that retain Fc binding while markedly reducing non-specific adsorption and enhancing alkaline tolerance.
In addition, for special modalities such as Fab, IgM, or antibody fragments, Protein L (which recognizes the κ light chain) or IgM-specific affinity resins can be used for targeted separations.
Key Factors in Selecting Affinity Resins
Selecting the appropriate affinity resin is pivotal for the development and industrial scale-up of antibody purification processes. An optimal resin must deliver strong separation performance while ensuring scalability, long-term stability, and supply reliability.
1. Ligand Type and Binding Specificity
Different affinity ligands recognize distinct structural regions of antibodies, and selecting the appropriate ligand is the foundation for achieving highly selective capture.
Protein A: Binds to the Fc region of antibodies; suitable for IgG1, IgG2, IgG4, and most Fc-fusion proteins. It remains the most widely used ligand for antibody capture.
Protein L: Recognizes the κ light-chain region; applicable to antibodies or fragments lacking an Fc structure (e.g., Fab, scFv), as well as Fc-engineered antibodies and some IgA or IgM types.
Protein G: Exhibits a broader binding range and serves as a complementary ligand to Protein A, suitable for special subclasses or certain Fab molecules.
IgM ligand: Recognizes polymeric structural domains and maintains high selectivity under high molecular-weight conditions.
Different antibody types (IgG, IgM, IgA, bispecific antibodies, antibody fragments, etc.) require ligand selection based on their binding structure, subclass, and molecular characteristics to achieve optimal purity and recovery.
The figure below (Fig. 2) presents a decision flowchart for selecting antibody affinity resins, helping R&D teams quickly determine suitable ligand types and recommended products at early stages.
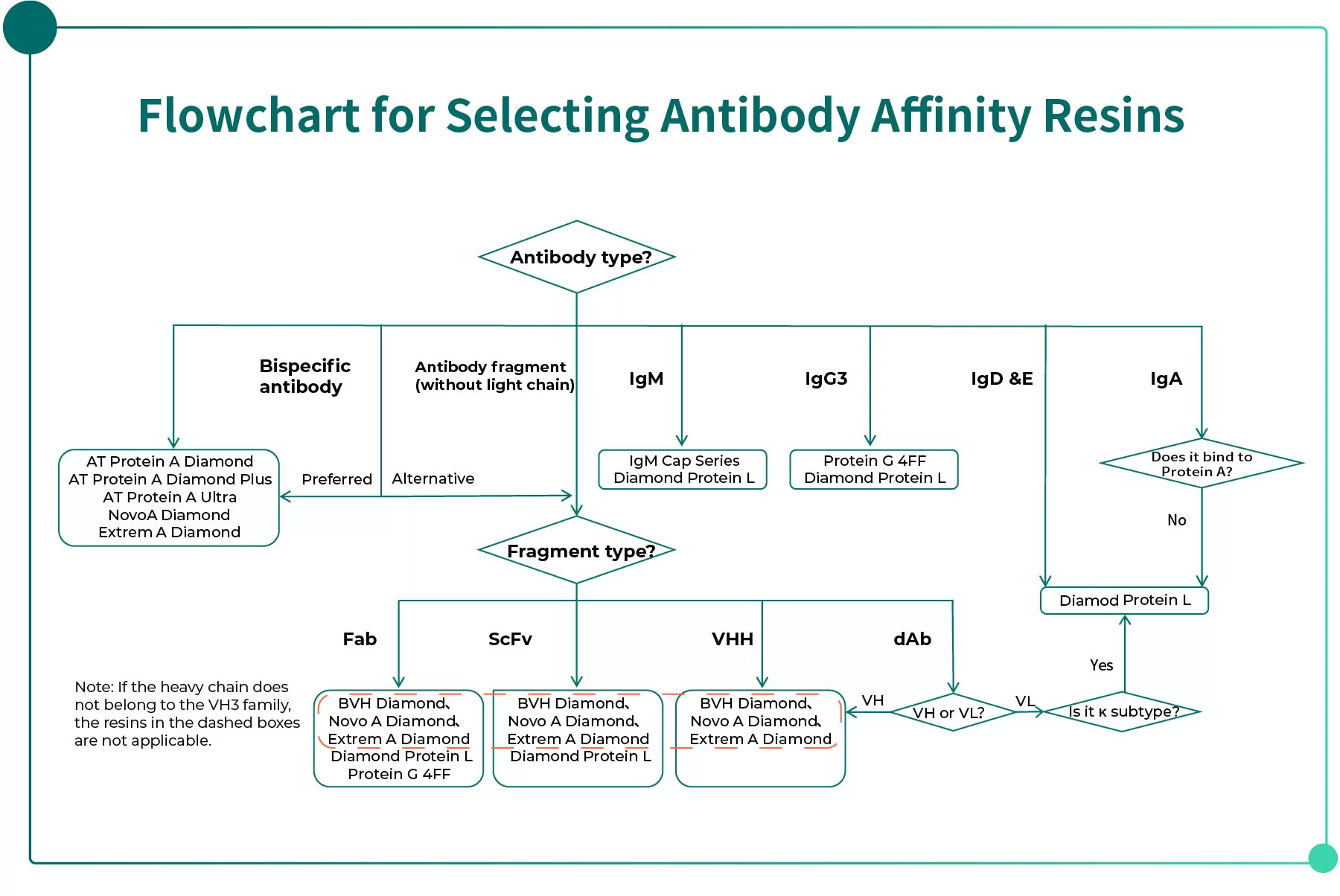
Fig. 2 Flowchart for Selecting Antibody Affinity Resins
2. Dynamic Binding Capacity (DBC) and Separation Trade-off
DBC reflects a resin’s binding capacity at specified flow rate and residence time, serving as a key metric for antibody capture efficiency.
· Higher DBC → greater capture throughput and processing capacity, but may lead to decreased resolution or to co-elution of impurities.
· Lower DBC → improved resolution but insufficient throughput.
Process development requires striking an optimal balance among load, flow rate, yield, and purity to maximize separation performance while controlling overall process economics.
3. Scalability and Pressure–Flow Performance
Scalability is critical for ensuring a smooth transition from lab to production scale.
Some resins perform well at small scale but, upon scale-up, inadequate mechanical strength or bed compression can lead to rising operating pressure, flow-rate limitations, and bed collapse—forcing mid- to late-stage process changes or reduced productivity.
It is advisable to evaluate pressure–flow behavior and the usable linear-velocity window early in process development to ensure that after scale-up, throughput and operating-pressure requirements can still be met.
4. Long-Term Alkaline Tolerance
Affinity chromatography need withstand repeated CIP with NaOH; alkaline tolerance directly determines resin lifetime and production cost.
Resins with high alkaline tolerance can endure hundreds of CIP cycles at 0.5–1.0M NaOH while maintaining binding capacity and performance, which is critical for long-term, stable manufacturing.
5. Lot-to-Lot Consistency and security of supply
At the production stage, chromatography resin is not only a key consumable for purification but also an essential element for stable and controlled manufacturing. Lot-to-lot consistency and security of supply directly affect process robustness and the consistency of final product quality.
· Lot-to-lot consistency: Evaluate whether critical performance remains stable across lots to secure repeatability and product-quality consistency over different production cycles. Excessive lot variation may cause fluctuations in recovery, shifts in impurity spectrum, or failures during scale-up validation.
· Security of supply: Assessment should cover not only the supplier’s continuity and reliability of delivery, but also long-term stability in quality systems, production capacity, process-consistency verification, and technical support. For commercial manufacturing, a stable and sustainable supply chain is a prerequisite to maintain steady operations and keep risks manageable.
During resin selection, product lot-to-lot consistency and the supplier’s long-term delivery stability should be taken into consideration as part of the overall evaluation.
This impacts not only the reproducibility of material performance but also the robustness of the process and the stability of final product quality.
Application Case: Mild-Elution Performance of Novo-A Diamond
In antibody purification, low-pH elution may induce conformational changes and aggregation.
Using Novo-A Diamond, complete elution can be achieved at pH 4.0, with purity more than 94%, recovery above 95%, and a binding capacity of approximately 65 mg/mL (Fig. 3).
Experimental Conditions:
Column: EzScreen 4.4 mL (bed height = 10 cm)
Sample: mAb, concentration 6.8 mg/mL
Binding buffer: 20 mM Tris-HCl, 0.15 M NaCl, pH 7.2
Wash buffer 1: 20mM NaAc,1 M NaCl, pH 7.2
Wash buffer 2: 20mM Tris-HCl, pH 7.2
Elution buffer 1: 50mM NaAc-HAc, pH 4.0
Elution buffer 2: 50mM NaAc-HAc, pH 3.5
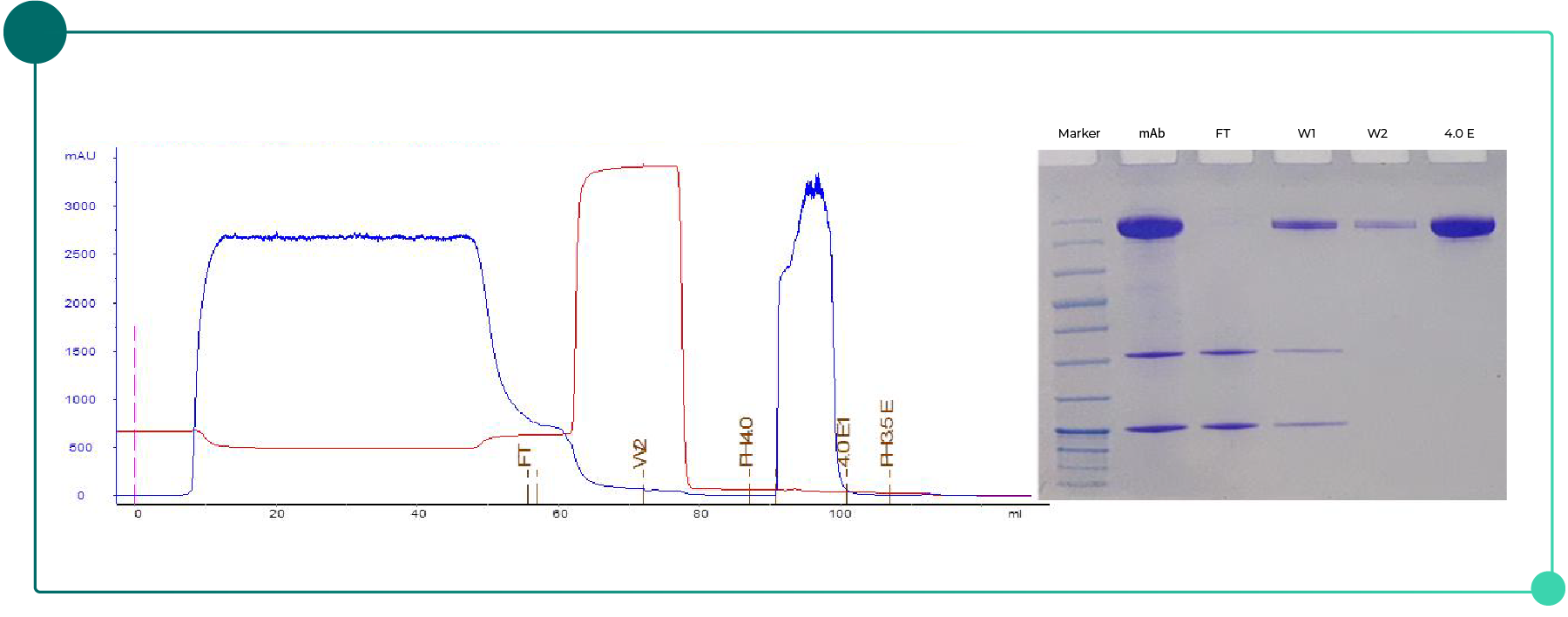 Fig. 3 Chromatogram and SDS-PAGE results for mAb purification with Novo-A Diamond.
Fig. 3 Chromatogram and SDS-PAGE results for mAb purification with Novo-A Diamond.
The results show that Novo-A Diamond maintains high purity and yield under mild elution conditions, making it suitable for antibodies sensitive to low-pH exposure.
Conclusion
Affinity chromatography is pivotal for downstream purification of monoclonal antibodies and related formats. The ideal affinity resin balances separation performance (DBC and purity), scale-up compatibility (pressure–flow behavior and alkaline tolerance), and supply reliability (lot-to-lot consistency and supplier capability).
A thorough assessment of these dimensions during early process development helps avoid late-stage changes and validation risks caused by flow limitations, ligand degradation, or supply risk.
Order Information
|
Product Name |
Pack Size |
Cat. No |
|
AT Protein A Diamond Plus |
25mL |
AA402305 |
|
AT Protein A Diamond Ultra |
25mL |
AA05701 |
|
Novo-A Diamond |
25mL |
AA05001 |
|
Extrem A Diamond |
25mL |
AA04501 |
|
Diamond Protein L |
25mL |
AA05101 |






.png)