Purification of Antibody Fragment by Diamond Protein L Affinity Resin
Isolated from bacteria Peptostreptococcus magnus, Protein L is a cell surface protein interacting with the variable regions of antibody Kappa light chain, specifically K1,K3 and K4 light chain, which enables its bind with about 67% of human immunoglobulins and 99% of mouse immunoglobulins.
Diamond Protein L is a novel affinity chromatography resin obtained by coupling Protein L ligands on highly rigid agarose beads. Compared to conventional resins, Diamond Protein L enjoys advantages including fast flow and low back pressure. Due to the strong affinity between protein L ligand and the variable regions of antibody Kappa light chain, Diamond Protein L is suitable in capture antibody fragments from large-scale cell culture medium, including F(ab‘)2, Fab, Single-chain fragment variable(scFv)and half-antibody fragment.
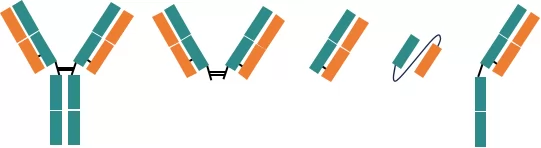
Fig.1 IgG antibody and antibody fragments
Characteristics
Highly rigid agarose beads, high flow rate, enhanced productivity
High specific Kappa light chain, effective capture of antibodies and antibody fragments with wide selection
High binding capacity, reduced processing time and resin consumption, lower production cost
|
Appearance |
White slurry |
|
Matrix |
Highly rigid agarose |
|
Average particle size+ |
~80μm |
|
Functional group |
Recombinant Protein L (from E.coli) |
|
Cross-linking method |
Epoxy chemistry |
|
Dynamic binding capacity++ |
~40 mg Human IgG/mL packed resin(residence time 6min) |
|
Chemical stability |
Common aqueous solutions: 10mM HCl,0.1M citric acid(pH3),6M Urea, 6M GuHCl, 30% isopropanol, 20%ethanol |
|
Max. pressure |
0.5MPa |
|
Pressure flow velocity |
≥300cm/h(2bar, bxk300/500, H=20cm) |
|
pH stability |
2~10(working), 15mM NaOH (CIP) |
|
Storage+++ |
2~8℃, 20% ethanol or 2% benzyl alcohol |
+ Particle size is normally distributed, average particle size is the medium value of the particle size in resin.
++Flow velocity 100cm/h, bed height 10cm, Buffer solution: 20mM PB, 0.15M NaCl, pH7.2.
+++2% benzyl alcohol is only used for international transport or special requirements from customer
Application case—half-antibody fragment purification
Resin: Diamond Protein L
Sample and sample loading volume:half-antibody fragment,30mg/mLresin
Equilibration buffer:20mM Tris-HCl, 0.15M NaCl, pH7.2
Elution buffer:50mM Gly-HCl, pH2.9
CIP buffer: 15m M NaOH
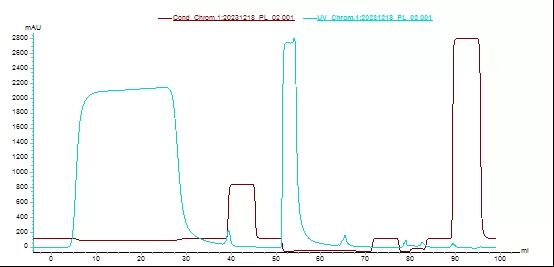
Fig.2 Chromatogram of Diamond Protein L resin
|
Sample |
Elution volume
(CV) |
Yield
(%) |
SEC% |
|
Aggregate |
Monomer |
Fragment |
|
Eluted products |
2.68 |
72.19 |
16.76 |
80.46 |
2.78 |
Recommendations for user
l Buffer selection
Binding buffer: usually choose neutral buffer, such as 20mM PB 0.15M NaCl pH7.2
Elution buffer: choose buffer with low pH, such as 0.1M sodium citrate (pH2.0-3.5)
l Increase purity of eluted products: During elution process, it is possible to get the optimized elution pH range by gradient pH elution. For the challenging antibodies or antibody fragments, it is possible to further remove product-related impurities including aggregates in eluted products via optimized elution concentration.
l CIP: In case of unsatisfying yield, it is recommended to conduct regeneration before CIP. Before doing CIP with NaOH, it is recommended to equilibrate column by
Solution (neutral pH), to prevent temperature soar in column due to direct contact between low pH buffer and high pH NaOH solution.
Order information
|
Resin |
Cat.No |
Pack size |
|
Diamond Protein L |
AA05101 |
25mL |
|
AA05102 |
100mL |
|
AA05103 |
500mL |
|
AA05104 |
1L |
|
AA05105 |
5L |
|
AA05106 |
10L |
Reference
[1] Serene W. Chen, Darryl Tan, et.al (2020) Investigation of the effect of salt additives in Protein L affinity chromatography for the purification of tandem single-chain variable fragment bispecific antibodies, mAbs
[2] Chen Chen, Tetsuya Wakabayashi et.al(2019) Controlled conductivity at low pH in Protein L chromatography enables separation of bispecific and other antibody formats by their binding valency, mAbs








.png)


