Purification Process of Herpes Zoster Vaccine
Introduction of herpes zoster
Herpes zoster(HZ) is an acute infectious skin disease caused by the reactivation of Varicella-Zoster Virus (VZV) incubating in the Dorsal root of spinal cord or cranial ganglion. Varicella-Zoster Virus, also known as Human herpesvirus 3(HHV-3), is double-stranded DNA virus with the smallest size (125kb in length)of all the herpes virus that are known to humans.
Since no specific drug for VZV infection is currently available, vaccination is an active precautionary measure with effectiveness. Several Herpes zoster vaccines have been approved by several countries since 2006, for the prevention of Herpes zoster and its complications.
Downstream purification process of herpes zoster
• Attenuated live vaccine:
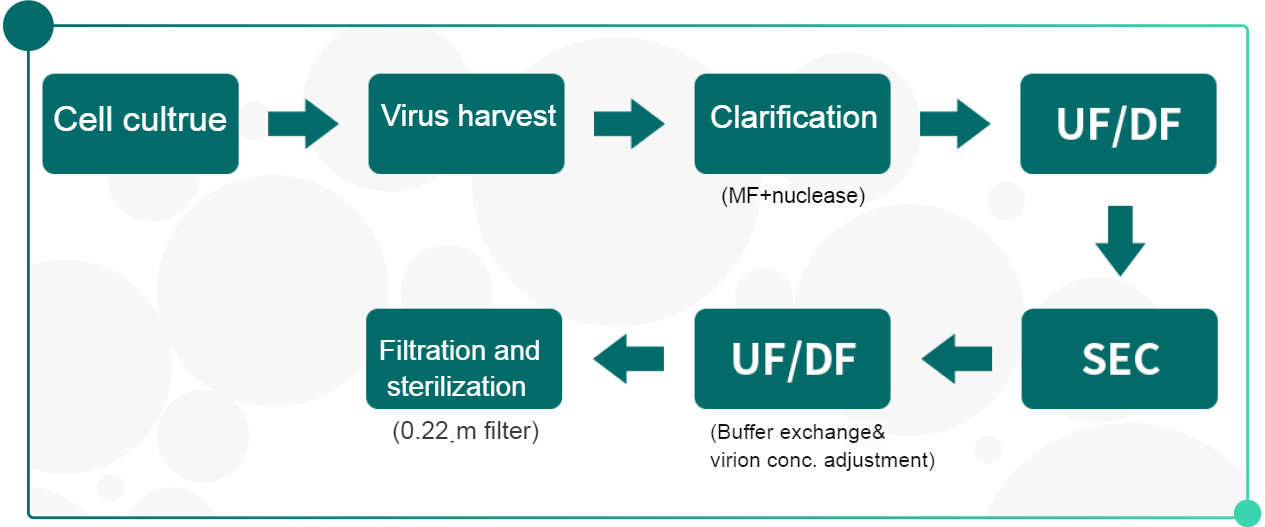
• Recommbinant protein vaccine:
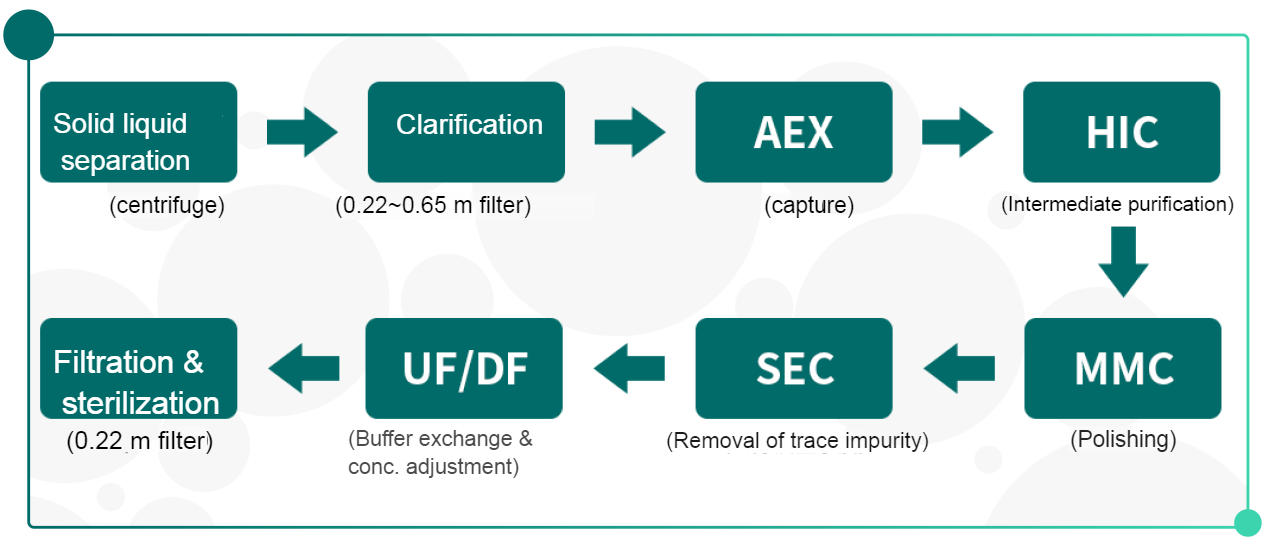
Case study: recombinant herpes zoster vaccine purification with Bestchrom resins
Bestchrom has successfully provided downstream purificiaiton solutions for multiple recombinant herpes zoster vaccines, including purification process development and allied resins. The following case study will show the MMC+HIC+AEX+MMC four-step chromatography approach, which will lead to SEC purity higher than 97% and HCP residual lower than 300ppm(Cygnus assay kit) in accordance with quality standards. Compared with conventional purification methods, this four-step approach can complete downstream purification process of herpes zoster vaccine without the involvement of SEC step.
1. Capture stage
Mixed mode chromatography (MMC) resin is used in the first capture stage. MMC resin combines various chromatography interactions, including IEX, hydrogen-bond and HIC interactions. Thus, purity of sample can be above 90% after being purified by MMC resin.
In addition, MMC resin enjoys certain tolerance towards saline ions, which means sample salinity under 30 mS/cm will generally exert no negative impact on its adsorption. That makes dilution ration reduction in corresponding samples possible, bringing down time consumption and manufacturing cost in the process.
Capture step removes a multitude of impurities and bring down HCP contents. In this case study, Diamond MIX-A resin was used for the purification of herpes zoster vaccine.
Resin: Diamond MIX-A
Sample: Recombinant Herpes zoster vaccine fermentation broth supernatant
Chromatography column/bed height: BXK 16/20, bed height 10cm
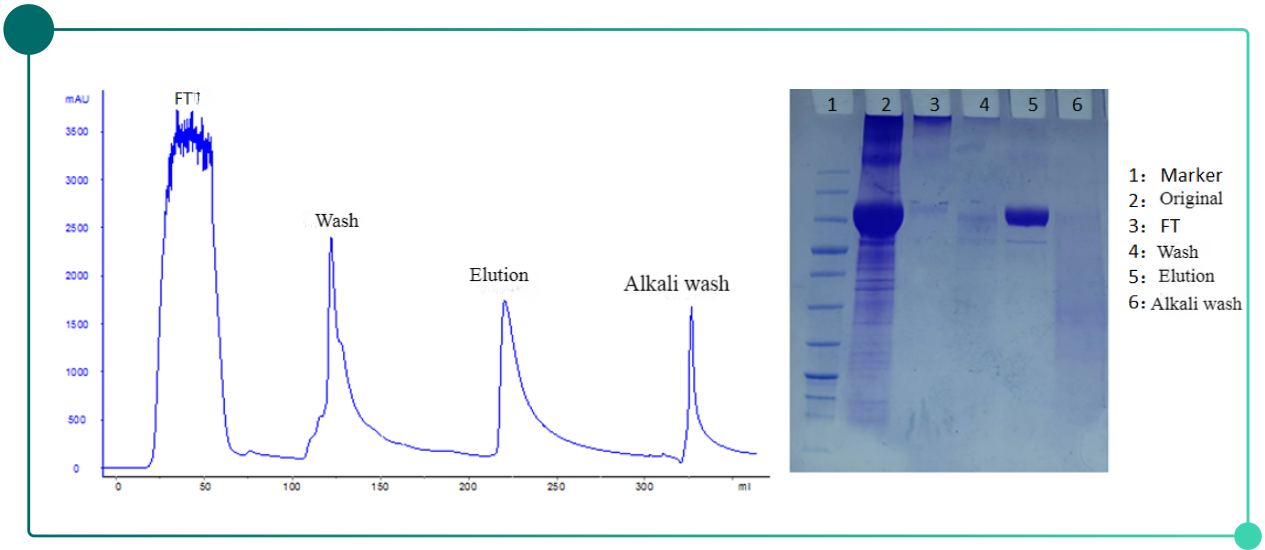
Fig.1.Chromatogram(left) and SDS-PAGE results(right) of herpes zoster vaccine purification with Diamond MIX-A
Purification results: According to the SDS-PAGE elextrophoretogram, no significant impurity band shows in the lane of sample No.5 apart from target protein. SEC purity >90 %, HCP residual reduced by 28 about times to thousands of ppm from its original more than a hundred thousand ppm.
Table 1.Diamond MIX-A technical parameters
|
Resin |
Matrix |
Resin type |
Ionic capacity |
Particle size |
Max Pressure |
Max flow rate |
pH stability |
|
Diamond MIX-A |
Highly rigid agarose |
Mixed mode AEX |
90~120 μmol Cl-/mL resin |
75μm |
5bar |
1200cm/h(0.1MPa, BXK50/30, h=15cm, 25℃) |
2~14(short term) 3~12(long term) |
2. Intermediate stage of purification
HIC resin is used in the second step (intermediate stage of purification). The key to choose HIC resin is the ligands with appropriate hydrophobicity. Order of ligand hydrophobic
ity: Butyl-S<Butyl<Octyl<Phenyl. HIC resin usually adopts the binding-elution mode, load sample at high salinity and elute sample by gradient elution (gradually reduce salinity). As ionic strength decreases, hydrophilic region of molecules exposure increases and molecules are eluted from column according to the increasing hydrophobicity.
Intermediate purification further removes sample impurities and reduces HCP residual. In this case study, Diamond Butyl Mustang was used for the purification of herpes zoster vaccine.
Resin: Diamond Butyl Mustang
Sample: Diamond MIX-A eluted sample
Chromatography column/bed height: BXK16/20, bed height 10cm
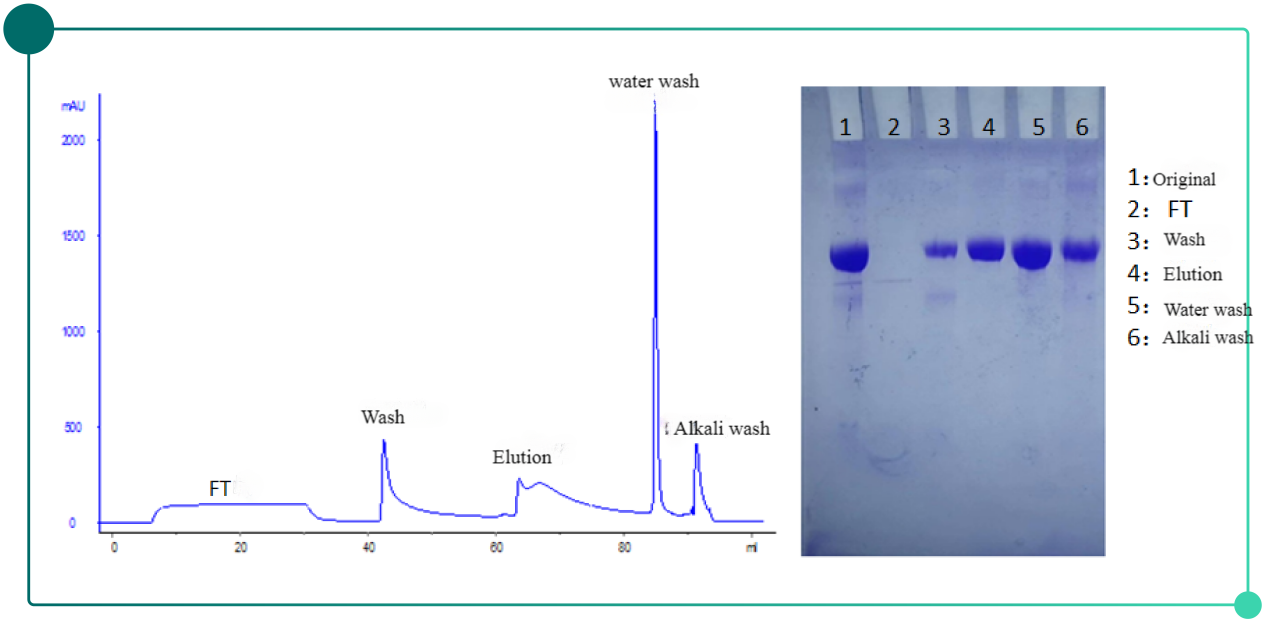
Fig.2. Chromatogram(left) and SDS-PAGE results(right) of herpes zoster vaccine purification with Diamond Butyl Mustang
Purification result: SEC purifity increases by 3%, HCP residual reduces to roughly 2000ppm.
Table 2.Diamond Butyl Mustang technical parameters
|
Resin |
Matrix |
Functional groups |
Average particle size |
Max Pressure |
Max flow rate |
pH stability |
pH stability |
|
Diamond Butyl Mustang |
Highly rigid agarose |
butyl |
36~44μm |
5bar |
200cm/h(0.15MPa, BXK 100/500, h=10cm, 20℃) |
3~13(working)
2~14(CIP,short term) |
2~14(short term) 3~12(long term) |
3. Polishing stage
AEX+Mixed mode strong AEX resin is used in the third step(Polishing step). AEX resin is coupled with functional groups with positive charges(Quaternary ammonium, diethylaminoethyl). It can bind with impurities with negative charge including endotoxin, HCD,HCP. Therefore, AEX resin is a key step in the removal of impurities such endotoxin.
In this case study, Diamond Q and Diamond MIX-A Mustang resins are used in the purification of Herpes zoster vaccine. Compared with Diamon MIX-A, Diamond MIX-A Mustang enjoys finer bead size and higher resolution.
Resin: Diamond Q
Sample: Diamond Butyl Mustang eluted sample
Chromatography column/bed height: EzScreen 4.9mL, bed height 10cm
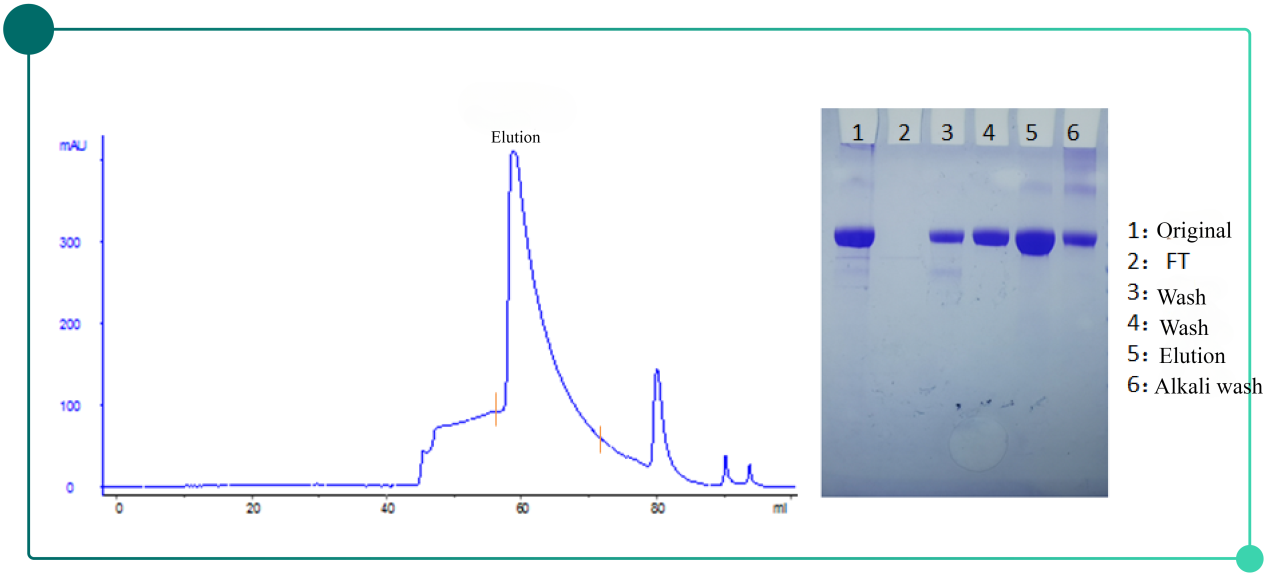
Fig.3. Chromatogram(left) and SDS-PAGE results(right) of herpes zoster vaccine purification with Diamond Q
Purification results: SEC detection purity increases dramatically , HCP residual drops below to hundreds of ppm.
Resin: Diamond MIX-A Mustang
Sample: Diamond Q eluted sample
Chromatography column/bed height: EzScreen 4.9mL, bed height 10cm
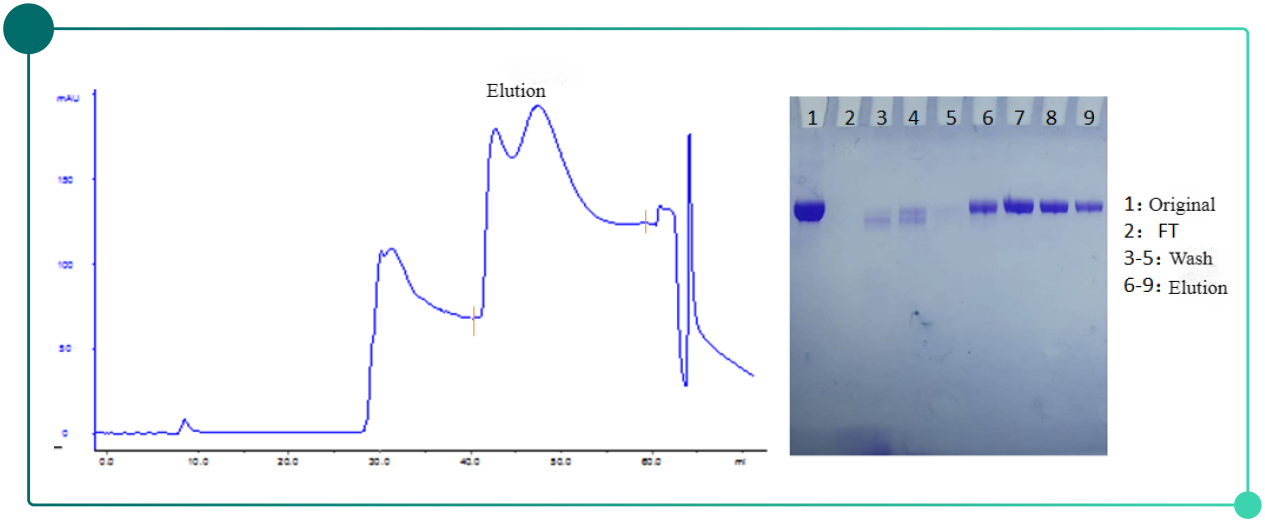
Fig.4. Chromatogram(left) and SDS-PAGE results(right) of herpes zoster vaccine purification with Diamond MIX-A Mustang
Purification results: SDS-PAGE elextrophoretogram shows the clean lane of eluted sample with no noticeable impurity bands. SEC purity can be higher than 97% and HCP residual can drop below 200ppm.
Table 3.Technical parameters of Diamond Q and Diamond MIX-A technical parameters
|
Resin |
Matrix |
Functional groups |
Ionic capacity |
Average particle size |
Max Pressure |
Max flow rate |
pH stability |
|
Diamond Q |
Highly rigid agarose with dextran chain |
Quaternary ammonium |
160~220 μmol Cl-/mL resin |
90μm
(45-165μm) |
5bar |
1500cm/h(0.5MPa, BXK100/500, h=20 cm, 20℃) |
2~14(short term) |
|
Diamond MIX-A Mustang |
Highly rigid agarose |
Mixed mode
Strong AEX |
80~110 μmol Cl-/mL resin |
36~44μm |
5bar |
450cm/h(0.5MPa BXK100/500, h=10cm, 20℃) |
2~14(short term)
3-12(long term) |
Conclusion
After going through the four-step chromatography purification approach with Bestchrom resins, herpes zoster vaccine can have a final SEC purity >97 %, HCP residual lower than 200ppm. In addition, final yield, purity and HCP residual are all in compliance with quality standard from customer.However, quality requirements can vary from vaccines for different types of herpes zosters, which presents diverse requirements to properties of resins used. Apart from products listed on our catalogue, customization service is also available in Beschrom, which will provide effective solutions to problems faced by customers in chromatography downstream processing, manufacturing and process optimization.








.png)


