Purification strategy for oligonucleotide drugs
Introduction
Small nucleic acid drugs(oligonucleotide drugs) belong to the most rapidly developing gene therapies at present. The mechanism of action is to directly target the pathogenic target gene or RNA fragment, which is usually synthesized by solid phase phosphorite chemical method. They are drug types consisting of 12-30 nucleotide single or double strands. The transcription and translation of pathogenic genes can be regulated by directly targeting pathogenic target genes or acting on messenger RNA(mRNA) in a high specificity fashion.
Due to the limitations of synthesis efficiency and raw material quality, not all oligonucleotides can be synthesized into a complete oligonucleotide strand. Initial product will contain various impurities, among which, most important one is caused by failed sequence (N-x/N+x) due to difference in single or multiple oligonucleotides. It is very important to notice that n-X and N+x impurities show very similar property as target molecules in terms of molecular size, charge property and hydrophobicity, bringing new challenges to purification process. This means it is necessary to adopt high resolution purification method after synthesis to effectively remove impurities from target molecules.
Therefore, the selection of resin and purification process will be vital to product quality and easy scale-up after the oligonucleotide synthesis. Currently, mainstream purification process uses anion exchange chromatography(AEX), reversed phase chromatography(RPC) and hydrophobic interaction chromatography(HIC). Due variety in nucleotide sequence and impurity content in crude products, it is possible to choose one or two items from the above-mentioned combination for purification process.
1. Classification of oligonucleotide drugs
Oligonucleotide drugs refer to chemical synthesized drugs composed of 12-30 nucleotide single or double strands. The drugs include antisense oligonucleotides (ASO), small interfering RNA (siRNA), microrna (miRNA), small activating RNA (saRNA), messenger RNA (mRNA), RNA Aptamer and so on.
The difference between oligonucleotide drugs and protein-based & small molecule drugs lies in its working mechanism. That is, oligonucleotide drugs can directly control gene expression rather than via through protein, and therefore are regarded as the third generation innovative drugs after small molecule and protein based drugs.
2. Synthesis of oligonucleotide drugs
Currently, mainstream methods for synthesis of oligonucleotide drugs include liquid phase synthesis, solid phase synthesis and biosynthesis. Among which, solid phase synthesis is the most popular method, which adopts solid phase phosphoramidite chemical methods for the synthesis.
Fig 1 shows monomer for synthesis, which is mainly composed of base, deoxyribose, 5'-DMT, 3' terminal 2-cyanoethyl and diisopropylamine. However, it is necessary for 2'-OH in phosphoramidite monomer to be protected to prevent cross reaction between 2'-OH and 5'-OH in the solid phase synthesis. The concerned protecting groups are generally TBDMS.
Besides, due to the existence of adenine, cytosine and guanine and some artificial bases in the primary amino group, it is necessary to use protecting groups in unit for protection purpose.
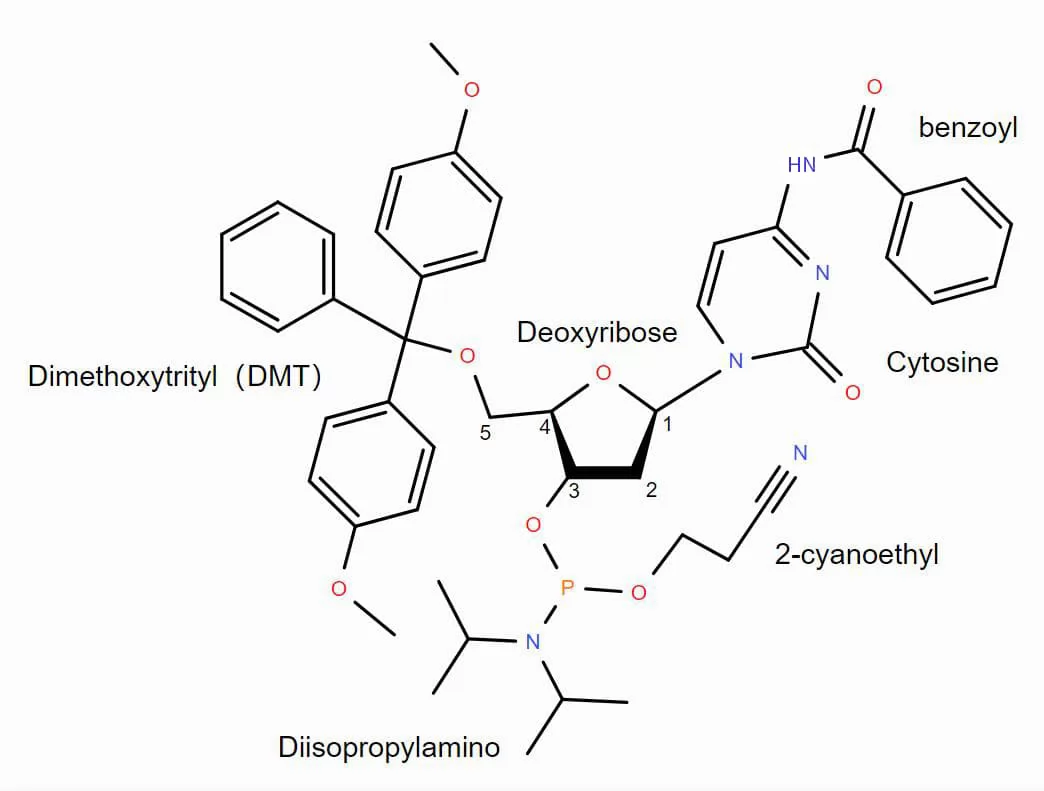
Fig.1 Phosphoramidite monomer in DNA
Solid phase synthesis of oligonucleotide drugs consists of 4 steps, namely de-protection, coupling, oxidation and capping. After the synthesis cycle, it is necessary to conduct ammonolysis on intermediate products by ammonia water, which cuts oligonucleotide from solid phase carrier and perform de-protection on groups.
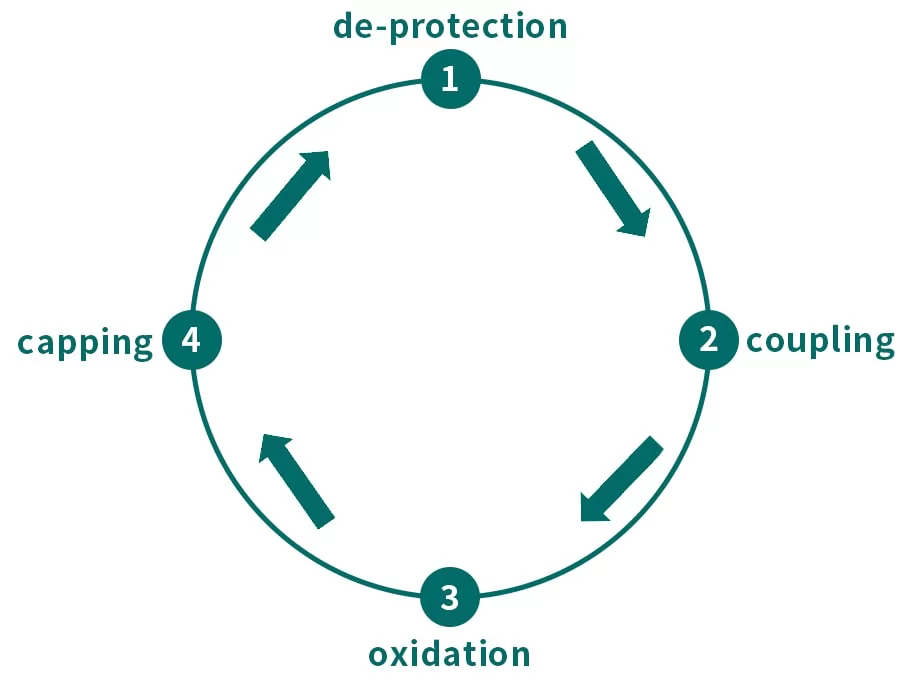
Fig.2 Solid phase synthesis of oligonucleotide
(1) De-protection: remove the 5'-DMT protecting groups from oligonucleotide by using dry organic solution and acid solution (dichloroacetic acid), which will expose 5'-OH for subsequent coupling step.
(2) Coupling: pump the activated monomers into synthesis column, de-protected 5'-OH Active will interact with intermediate of phosphorite tetrazolium and form new phosphorus-oxygen bond, which will extend nucleotide chain.
(3) Oxidation: The phosphorous ester bond from previous coupling step, will easily be hydrolyzed by acid or alkali. Thus, it is necessary to perform oxidation or sulfuration so as to form phosphodiester (or phosphonic acid diester) bond.
(4) Capping: To prevent coupling interaction between the active 5’ groups and activated phosphorous ester monomers added in subsequent cycle, which will produce N-1 impurity, it is necessary to add caps on those 5’-OH hydroxyl groups which do not participate in coupling step.
3. Purification technology of oligonucleotide drugs
Being subject to reaction efficiency and raw material purity, various impurities might exist in synthesized product. Specifically, discrepancy(N-x/N+x) of single or multiple oligonucleotide, remains of protecting groups(e.g. DMT+, 3’+ products), as well as purine removals. Alterations in these groups or sequence will cause property difference between target molecules and impurities, specifically:
● Phosphate group: electrostatic charges of an oligonucleotide depends on the number of phosphoric acid or modification groups, bases number and the fact whether secondary structure shields charged groups. Generally speaking, it is common to extend structure by opening hydrogen bond while keep the base uncharged(pH>pKa) under denaturation condition (e.g. pH12) , by which means to effectively isolate N-1 impurities.
● DMT on/off: DMT is a strong hydrophobic groups, which can interact with RPC/HIC. Therefore, it can be used in the separation of DMT-carrying full-length sequences.
● Thiolation: when oxygen atoms in phosphate group is replaced by sulfur atom, polarity of negatively charged groups will increase, which leads to stronger binding with AEX resin under most pH conditions.
● Methylation: when oxygen atoms are replaced by methyl groups, hydrophobicity will increase.
Therefore, oligonucleotide drugs process usually achieves isolation purpose based on the above-mentioned property discrepancy. That is, a combination of IEX, HIC and RPC methods is usually adopted in the purification process.
Case study: Oligonucleotide purification by BestPoly 15Q resin
AEX is a chromatography method which can isolate oligonucleotide under water phase condition without the participation of organic solution. As a result, it is not essential to take explosion-proof condition of production workshop and production equipment into consideration.
BestPoly 15 Q is a strong AEX resin based on PSDVB backbone(matrix). With an average particle size of 15μm, the resin enjoys merits including excellent chemical stability, high rigidity and feasibility of operating under high pH condition(High pH condition can effectively prevent self-complementation of single strand and aggregation of GC-containing oligonucleotide ). In this case, by using BestPoly 15Q resin for the purification of oligonucleotide, up to 52% of final samples can reach a purity level of 90% or above.
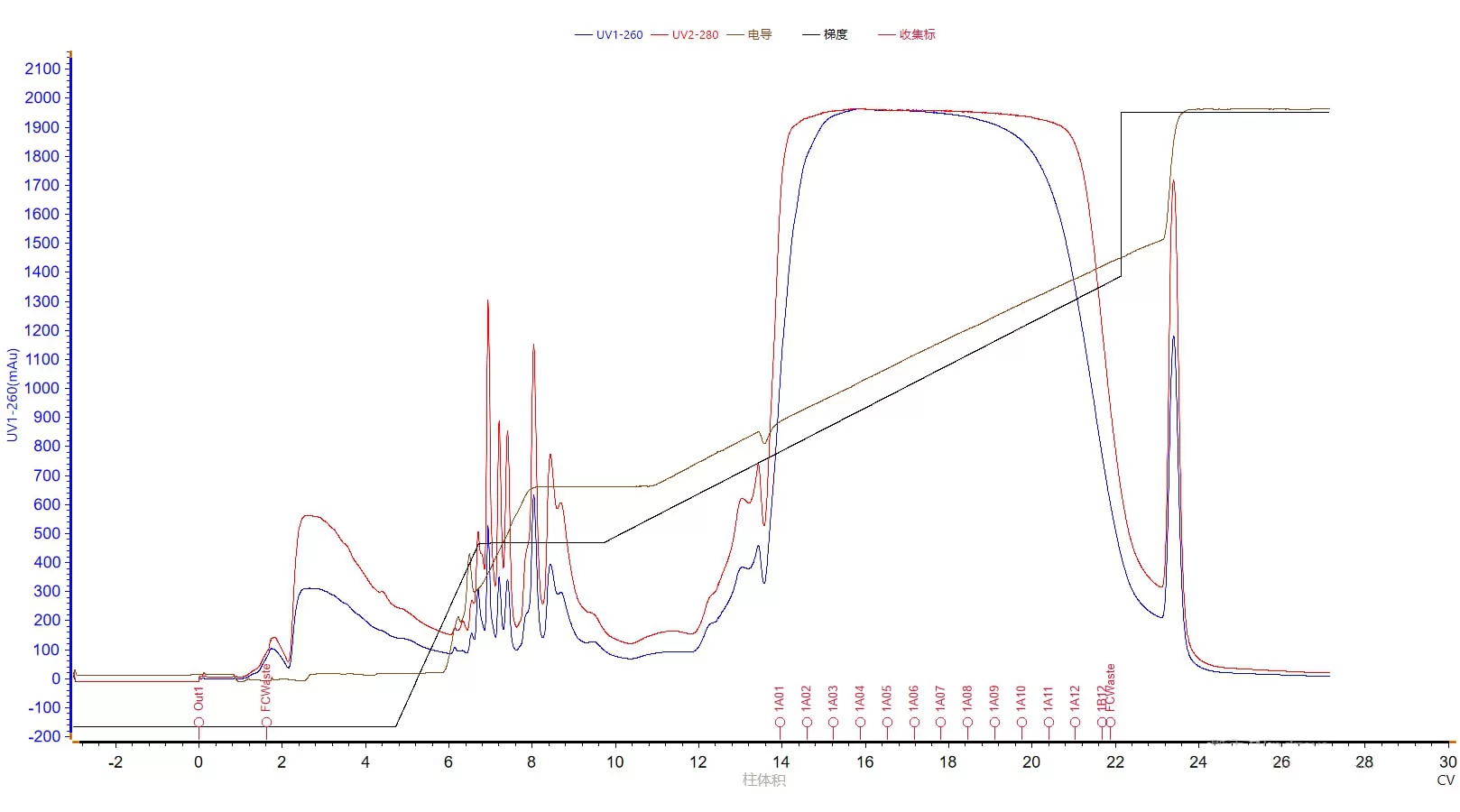
Fig.3 Purification of oligonucleotide by BestPoly 15Q resin
4. Conclusion
Oligonucleotide drugs, as a new biopharmaceutical R&D platform, has stepped to a phase of maturity and at the threshold of rapid development. Considering its ground-breaking technological advantages, it is possible for disruptive changes to take place in the mildest and strategy of chronic disease therapy. The transformation, which will very likely bring a major reconstruction in Monoclonal antibodies industry, opens a ten or twenty-year- window period of for oligonucleotide drugs sector.
For the production of oligonucleotide drugs, the synthesis step and subsequent purity are most important. Therefore, the selection of proper resin with excellent efficiency is the key. Bestchrom, as a reliable chromatography solution provider, will persistently expand product range to better meet customer purification needs.
Reference:
[1] Ramasamy T, Ruttala HB, Munusamy S, Chakraborty N, Kim JO. Nano drug delivery systems for antisense oligonucleotides (ASO) therapeutics [published correction appears in J Control Release. 2023 Feb;354:34]. J Control Release. 2022;352:861-878.
[2] Jamil Shanagar. Purification of a synthetic oligonucleotide by anion exchange chromatography: Method optimisation and scale-up. J. Biochem. Biophys. Methods 64 (2005) 216–225








.png)


