Bispecific antibodies (BsAbs) enjoys great potential in tumor immunotherapy as a result of their multi-target mechanism. By the end of 2025, a total number of 19 BsAb-based drugs have been launched with more than 300 clinical trials being conducted worldwide.
In light of huge therapeutic potential, more than 50 types of recombinant bispecific antibody structures have been developed. In terms of structure, They can be categorized into Fc-depleted bsAbs(Non IgG-like BsAb) and Fc-containing bsAbs(IgG like BsAb). The latter have symmetric and asymmetric types.
However, the structural complexity of BsAbs means large amount of product-related impurities. For example, homodimer, LC-missing byproduct,half antibody and aggregates.These above-mentioned impurities usually have similar physical and chemical property compared with target molecules, making effective separation difficult to achieve by commonly used IEX chromatography.
Mixed-Mode Chromatography, as an innovative resin combining multiple interactions, has been playing a key role in the purification of BsAbs.
What is Mixed-Mode Chromatography?
Mixed-mode chromatography resins by Bestchrom (take Diamond MMC Mustang) possess the following mechanisms:
Weak cation exchange interaction
Hydrogen bond interaction
Hydrophobic interaction
Can be widely applicable for the removal of complex impurities including aggregates, antibody fragments, LC-missing byproduct, half antibody and homodimer.
Application cases
The removal of aggregates and antibody fragments
-
Molecule:Bispecific antibody
-
Sample:Protein A eluate (pH adjusted to 5.5)
-
Buffer:50 mM NaAc-HAc, pH 5.5;50 mM NaAc-HAc, 1 M NaCl, pH 5.5
-
Load sample volume:35 g/L and 80 g/L
-
Gradient:0-100% B 20CV increasing salt linear gradient
Result: Diamond MMC Mustang enjoys stable resolution under different binding capacity, which means comparable performance to global branded resin.
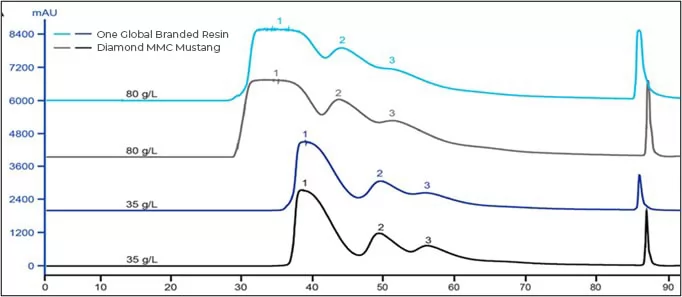
Fig.1 Chromatogram of Diamond MMC Mustang and one global branded resin
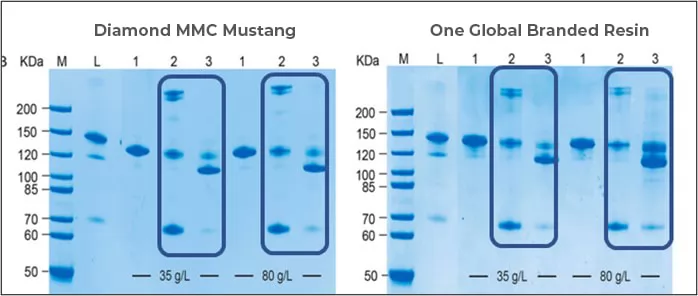
Fig.2 SDS-PAGE of samples after being purified by Diamond MMC Mustang and global branded resin
The removal of LC-missing byproduct
-
Molecule:Symmetric WuXiBody
-
Separation principle:pI disparity(the gap between BsAb and LC-missing byproduct is 0.21)→different binding strength →successful separation
-
Sample:Protein A eluate (pH adjusted to 6.0)
-
Buffer:40 mM phosphate buffer, pH 6.0;40 mM phosphate buffer, 150 mM NaCl, pH 6.0
-
Gradient:0-100% B 20CV increasing salt linear gradient
Result: Achieve the separation of BsAb and LC-missing byproduct. According由to SDS-PAGE, peak tail is LC-missing byproduct
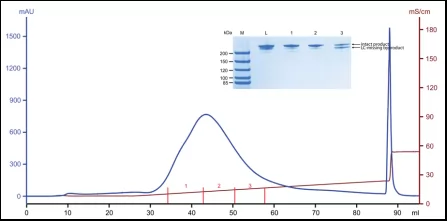
Fig.3 Purification of LC-missing byproduct and quality control using mixed-mode cation resin.
The removal of half-antibody and homodimer using linear pH gradient
-
Molecule:Bispecific antibody(Asymmetric IgG-like knobs-into-holes)
-
Separation principle:binding strength difference of half antibody, hole-hole homodimer, bsAb and aggregate on mixed mode cation resin.
-
Sample:Protein A eluate (pH was adjusted to 7.0 and (NH4)2SO4 was added to 1 M)
-
Buffer:20mM Na-citrate, 20mM NaH2PO4, 20mMTris, 20mM glycine, pH 5.5;20mM Na-citrate, 20mM NaH2PO4, 20mM Tris, 20mM glycine, pH10.0
-
Gradient:0-100% B 20CV increasing linear pH
Result:According to chromatogram and SDS-PAGE, half antibody, homodimer and aggregate have been successfully separated.
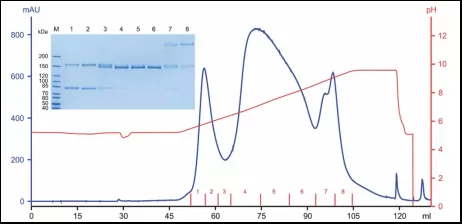
Fig.4 Purification of half antibody and homodimer using mixed-mode cation resin as well as quality control
Using hydrophobic interaction to remove antibody fragment and aggregate
-
Molecule:Bispecific antibody
-
Separation principle: bind at high salinity,elute at low salinity →remove protein fragment and aggregate
-
Sample:Protein A eluate (pH was adjusted to 7.0 and (NH4)2SO4 was added to 1 M)
-
Buffer:50 mM Tris-HAc, 1 M (NH4)2SO4, pH 7.0;50 mM Tris-HAc, pH 7.0
-
Load sample volume:30 g/L
-
Gradient:0-100% B 20CV decreasing salinity (1M (NH₄)₂SO₄ → 0)
Result: successfully separate protein fragment and aggregate, shows comparable performance to global branded resin.
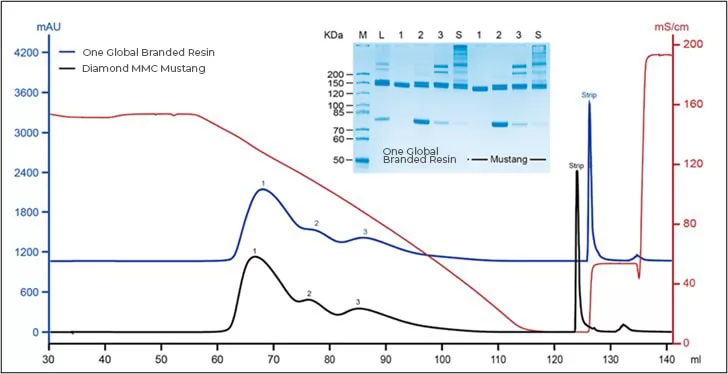
Fig.5 Purification of protein fragment and aggregate using mixed-mode cation resin as well as quality control
Diamond MMC Mustang lot-to-lot consistency evaluation
To evaluate lot-to-lot consistency of resin, three batches of Diamond MMC Mustang resins in the experiment have been packed in column. Purification procedure(under same condition) have been performed for three times.
-
According to the overlapped chromatograms, three batches of resins showed good reproducibility and excellent separation performance. The three batches showed high uniformity(see Fig.6).
-
DBC@5% breakthrough of three batches of resin were highly consistent (DBC@ 5% breakthrough of Batch 1,2,3 were 94mg/mL,94 mg/mL and 95mg/mL respectively).
-
Eluent mass and recovery of three batches showed consistency.
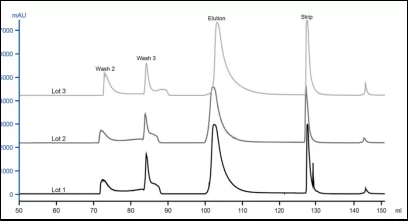
Fig.6 Chromatogram of three batches of Diamond MMC Mustang resin
Conclusion
Mixed-mode chromatography, as an indispensable method in downstream purification of BsAbs, can effectively remove impurities difficult to isolate by traditional methods based on its multiple interactions. It will guarantee quality improvement in addition to provide a new perspective to optimization of production process.
Reference
[1] Klein, Christian, et al. "The present and future of bispecific antibodies for cancer therapy." Nature reviews Drug discovery 23.4 (2025): 301-319.
[2] Li, Yifeng , et al. "A roadmap for IgG-like bispecific antibody purification." Approaches to the Purification, Analysis and Characterization of Antibody-Based Therapeutics (2020).
[3] Li, Yifeng . "A brief introduction of IgG-like bispecific antibody purification: Methods for removing product-related impurities." Protein Expression and Purification 155(2019):112-119.
[4] Chen, Serene W , and W. Zhang . "Current trends and challenges in the downstream purification of bispecific antibodies." Antibody Therapeutics 4.2(2021).
[5] Zhang, Shengwei , et al. "Evaluating a new mixed-mode resin Diamond MMC Mustang using Capto MMC ImpRes as a benchmark." Protein Expression and Purification 186(2021):105930.
[6] Tang, Jiaqin. , et al. "Removal of half antibody, hole-hole homodimer and aggregates during bispecific antibody purification using MMC ImpRes mixed-mode chromatography." Protein expression and purification 167(2020):105529.








.png)


