Strategy for effective HCP clearance in downstream processing of antibody
Background Information
In the downstream processing of recombinant antibody based drugs, removal of product-associated impurities including endotoxin, virus, HCP and HCD is always the key purpose.
Among all the impurities, HCP (host cell protein) is a complex endogenous protein complex with different physical and chemical properties produced by host cells expressing recombinant antibodies. Due to its immunogenicity and potential proteolytic activity, which is believed to reduce stability and efficacy of antibody drugs, HCP is considered a key quality parameter for antibody drugs. The clearance of HCP therefore, belongs to key objectives of downstream bioprocessing for antibody drugs. Generally speaking, acceptable range for residual HCP in antibody drugs bulk.
Take the Chinese hamster ovary cell (CHO) antibody as an example, HCP level can be reduced stepwise in chromatography processing procedures including Affinity chromatography, depth filtration, ion exchange chromatography and mixed-mode chromatography.
Affinity Chromatography
Affinity chromatography enjoys highly specific binding towards antibody and therefore belongs to the most commonly used approaches in the capture of antibody downstream processing. The high selectivity of affinity chromatography makes it an effective approach in HCP clearance.
Non-specific binding between HCP and affinity resins has very limited impact in the clearance of residual HCP. However, multiple binding sites exist between HCP and target protein based on non-specific binding, which will leads to simultaneous purification of the two substances, causing the presence of HCP in eluate.
To bring down the HCP content in sample to the lowest level, washing is usually performed before elution of target proteins. The key of washing is to damage interactions between HCP and affinity resin/antibodies. Usually buffers whose pH is between equilibration and elution buffers are chosen for washing (pH 5.0~6.5), in order to remove the non-specifically bound HCP. However, this strategy only has very limited effectiveness in the clearance of HCP which can interact with target protein. Meanwhile, low pH intermediate washing might adversely impact yield, especially at high volume sample loading.
Under such circumstances, it is possible to break the interaction between antibody and HCP via alkali buffer (pH 8.0~10.0) and therefore achieve the purpose of HCP clearance.
In addition, apart from optimization on buffer and pH during washing, adding salt and additives (e.g. urea, arginine, propylene glycol and triton X-100, etc.) can also break electrostatic interaction, hydrophobic interaction and hydrogen bonds between HCP and target protein. By washing, HCP bound on column can be removed and HCP level in eluate can be reduced dramatically.
Table 1. Commonly used additives and conc.
|
Additives |
Concentration |
|
Arginine |
0.1~1 M |
|
GdnHCI |
1M |
|
Isopropanol |
5~20% |
|
NaCI |
0.1~2 M |
|
Propylene glycol |
5~20% |
|
Triton X-100 |
≤1% |
|
Tween-80 |
1% |
|
Urea |
0.5~3 M |
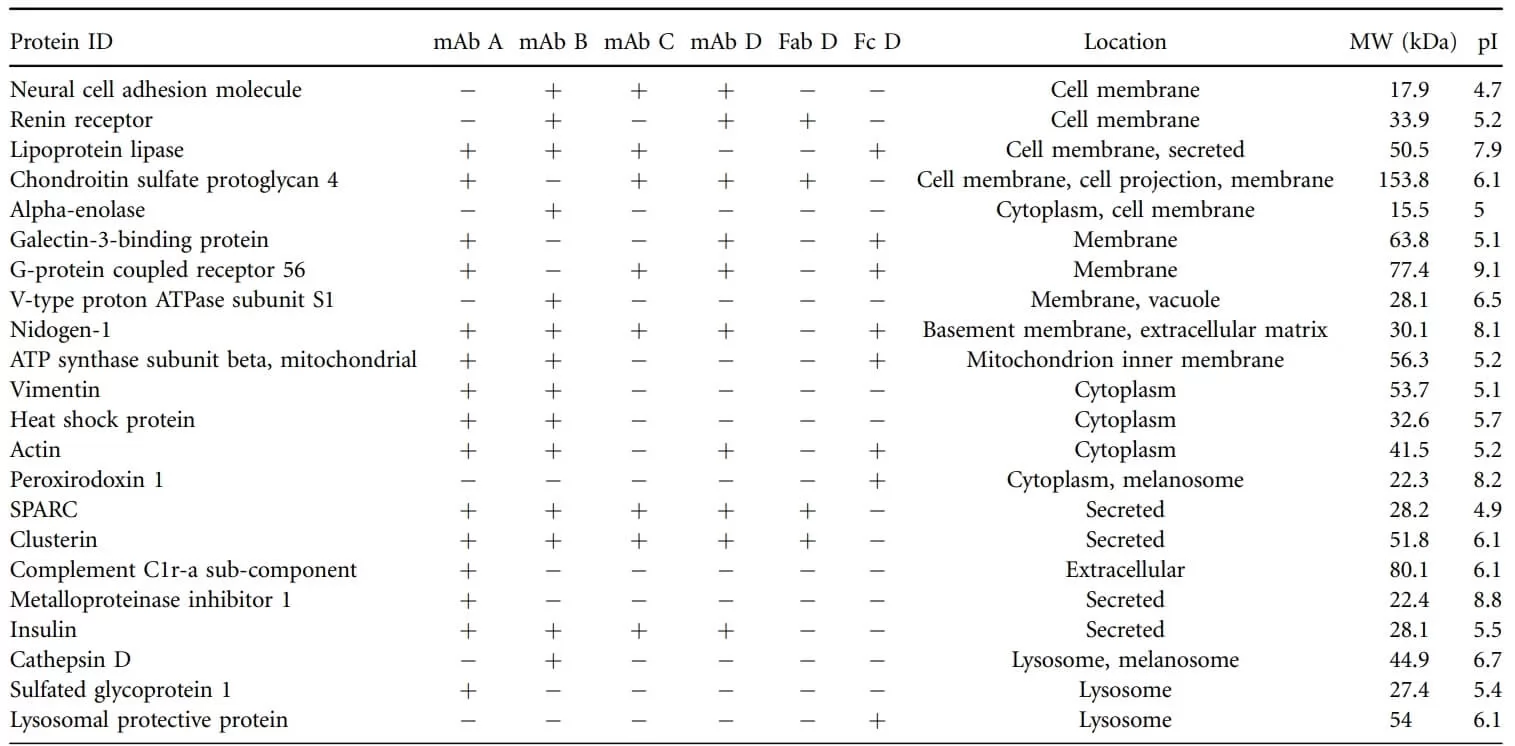
Table 2. Proteins which can strongly bound HCP in Fab and Fc domain of mAb
Depth Filtration
For samples eluted after affinity chromatography, after viral inactivation by low pH (3.5~3.8), it is essential to neutralize pH for convergence of subsequent steps. Most HCP (pI 4.5~7.0) will go beyond pI when adjusting pH and therefore reduce its solubility.
For the pH optimization after low pH viral inactivation, it is recommended to effectively aggregate or precipitate HCP via choosing the optimal neutralized pH. Additionally, it is possible to achieve maximum HCP precipitation by adding additives such as caprylic acid and polyethylene glycol, while minimizing loss of target substance at the same time. The processed feedstock will go through depth filter, which will adsorb HCP based on its property and maximize clearance of HCP.
Ion Exchange Chromatography
Affinity chromatography and depth filtration can usually remove more than 90% of HCP. However, it is still possible that target residue HCP requirement is not met after the above two steps, when polishing is required for the further clearance job. Ion exchange chromatography is mostly commonly used for that purpose.
Most antibodies have higher pI compared with HCP and therefore AEX resin can be chosen to remove HCP at F/T mode, that is loading sample at low pH(lower than pI of antibody) and low conductivity. Specifically, most HCPs have low pI, negatively-charged resin will bond such HCPs whereas positively-charged antibodies will not bind and flow through. This approach works effectively on isolating target antibody from HCP. Besides, it is possible to further optimize the purification process by adjusting load conditions including buffer types, ionic strength and pH. For weak AEX resins, recovery can be improved by other methods including adding sample loading volume and washing after load.
By contrast, CEX resins rely on B/E mode to remove product-associated impurities. Apart from buffer types, ionic strength and pH, other parameters such as load condition, washing strategy and elution condition are essential for both HCP removal and product quality improvement. In addition, it is also possible for CEX resins can remove HCP and aggregates by weak B/E mode.
Mixed-mode Chromatography
Mixed-mode Chromatography enjoys higher selectivity compared with single mode chromatography. Hydrophobic groups and ion charges on antibody surface have very different distribution from that of most HCP. Mixed-mode chromatography resins functions by combining hydrophobic interaction and ionic exchange interaction, which enjoys more excellent performance in the removal of impurities such as HCP compared to single-mode approaches. However, because of its inherent complexity of mixed-mode chromatography (interactions from multiple factors), a wider optimization is necessary for its better application.
For example, pH condition optimization can effectively control ionic interaction and binding capacity, improving final recovery. Meanwhile, optimization on conductance (loading, washing and salt concentration in elution) will determine the hydrophobic interaction at binding and elution. Apart from that, additives such as arginine can also improve isolation/selectivity of mixed-mode chromatography.
Conclusion
Effective removal of HCP is key parameters in antibody drug production, which is mainly dependent on strict control in upstream cell culture and downstream processing.
In the downstream processing of antibody drug, flexible combination of various purification methods is required for effective HCP clearance. To maximize HCP removal and improve processing stability, optimization on each key step is of key importance. In other words, a comprehensive application of various HCP clearance approaches will be crucial to ensure the stability and efficacy of antibody drugs.
Reference:
1. Levy, N.E., Valente, K.N., Choe, L.H., Lee, K.H. and Lenhoff, A.M. (2014), Identification and characterization of host cell protein product-associated impurities in monoclonal antibody bioprocessing. Biotechnol. Bioeng., 111: 904-912. https://doi.org/10.1002/bit.25158
2. Li, Yifeng. (2017). Effective strategies for host cell protein clearance in downstream processing of monoclonal antibodies and Fc-fusion proteins. Protein Expression and Purification, 134(), 96–103. doi:10.1016/j.pep.2017.04.006
3. X. Santarelli, C. Cabanne, Mixed mode chromatography: a novel way toward new selectivity, Curr. Protein Pept. Sci. 20 (2019) 14–21, Doi: 10.2174/1389203718666171024121137









.png)


