Strategy for effective removal of byproducts from BsAb
The structure of BsAb
Bispecific antibody (BsAb) is a type of artificial antibody via gene recombination technology. It can specifically bind two different antigens and epitopes at the same time. Based on its unique specificity and dual function, BsAb drugs have become a hot topic in Bio-pharmaceutical sector, particularly for its huge potential and broad perspective in oncology treatment, inflammatory disease and autoimmune disease and other related fields.
The strategy for downstream processing development of BsAbs is mainly developed on the basis of established mAbs purification method and references. This strategy is reliable for the similarity in structure of both types of antibodies, which making the mAbs purification approach an ideal starting point for BsAbs purification. Figure 1 illustrates the structure of IgG mAb, which consists of two heavy chains (HC) and two light chains(LG).
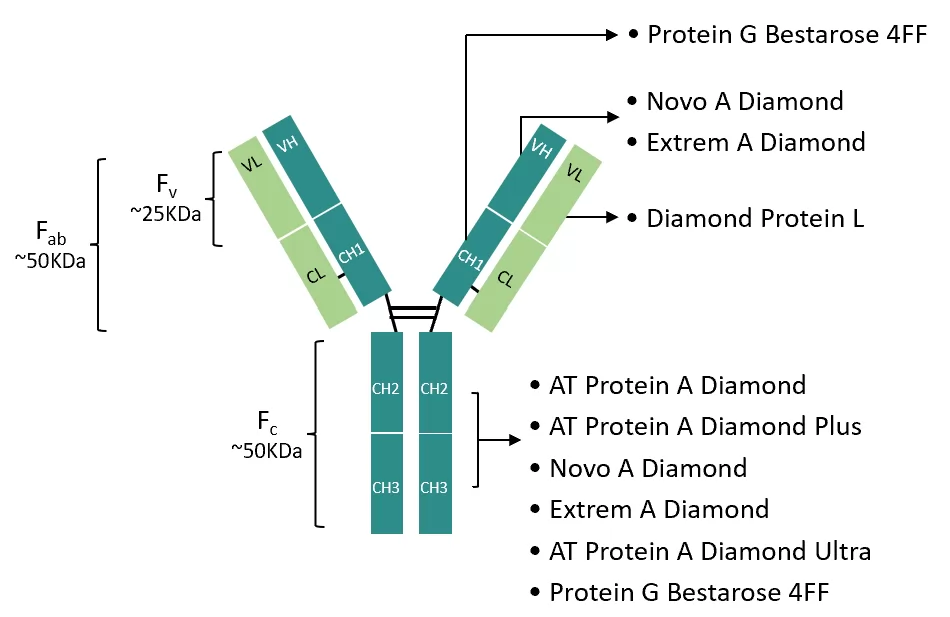
Fig.1 IgG mAb and its major binding sites for affinity ligands
Specifically, the heavy chain (HC) consists of multiple domains such as variable heavy chain (VH), constant region 1 (CH1), hinge region, constant region 2 (CH2) and constant region 3 (CH3). The light chain (LC) contains a variable light chain region (VL) and a constant light chain region (CL). Among them, VL pairs with VH domains to form the variable fragment (Fv) of the antibody, while VL, VH, CL and CH1 domains together form the antigen-binding fragment (Fab), which is a key region for the mutual recognition of antibodies and antigens.
On the other hand, the CH2 and CH3 domains combine to form the crystallizable fragment (Fc) region of the antibody, which is responsible for the effector function of the antibody. For example, its interaction with immune cells or the complement system.
Figure 1 specifies the binding site of IgG and affinity ligands, which are marked by arrows. These marked sites shall be the priority when setting purification strategy. By a deep understanding of structural details and binding sites, R&D staff can get a more focused strategy for purification of BsAbs and finally achieve the production of antibodies with high efficiency and purity.
Based on their structure, BsAbs fall into three categories, which is (i) asymmetrical (ii) symmetrical (iii) fragment-based BsAbs. Since their importance to downstream process strategy[1], the three types of antibodies have been shown in the following Figure 2.
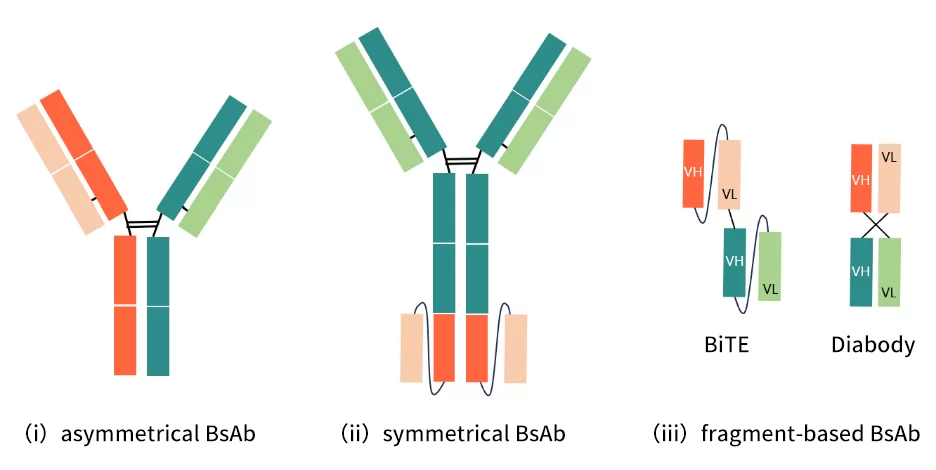
Fig.2 structure of three types of BsAbs
i Asymmetrical BsAb: This type of BsAb usually has heavy chain and light chain from two different parental mAbs. Among which, partial or all of light chain/heavy chain can be designed to be equal in two arms to minimize mismatch.
ii Symmetrical BsAb: it contains Fc fragments and has symmetrical domain sequence on both sides. It achieves multiple specificity by adding extra antigen recognition domains to antibody (e.g. scFv).
Iii Fragment-based BsAb: this type of BsAb is obtained by different connection methods of variable regions. For example, BiTE structure (one VH region of scFv connected to its own VL and VL of another scFv) and Diabody structure (two scFvs, which are respectively connected to VH and VL regions of each other, instead of connecting to their own VL and VH regions )
Major byproducts of BsAb
The complexity of BsAb structure contributes to the appearance of multiple byproducts, which will make the subsequent purification process more challenging. The causing and analysis of major by-products:
1. Homodimer: during cell culture, due to the co-expression of multiple HCs and LCs, BsAb is prone to HC and LC mismatch, leading to the production of enormous non-functional or single specific antibodies with similar sizes and properties. These antibodies are called homodimers. To lower mismatching, it is possible to boost the production of target BsAb via molecular design. For example, the widely used knob-into-hole(KiH)technology, which can remove partial but not all of the homodimers.
2. Fragment missing: BsAbs with missed HC or LC are the most common byproducts. For example, 1/2 BsAb (miss one HC and one Lc) and 3/4BsAb (miss one LC).
3. Aggregates: BsAb molecules have complex structure, various forms and special molecular structure, which leads to the occurrence of aggregates under the impact of different physical, chemical conditions and cell culture pressure. According to research results, fragment-based BsAb will more likely to produce aggregate compared to conventional IgG due to the missing of Fc region. In addition, symmetrical BsAbs are observed to have high tendency of aggregation (as high as 50% aggregates ratio). This tendency might be caused by the increased flexibility and length of chains, which leads to the higher possibility of intermolecular domain exchange.
Tips for the clearance/removal of the above-mentioned three by-products
1. The removal of Homodimer
Homodimer enjoys very similar physical and chemical property to BsAb molecules. Therefore, the setting of right purification strategy requires the consideration of difference in structure, affinity, charge property and hydrophobic interaction of monodimers. Refer to the following description for the case study of homodimer removal by using affinity, IEX and mixed-mode chromatography approaches.
Protein A/L affinity chromatography: Protein A can specifically bind Fc domain of IgG1 or IgG4. By adjusting binding capacity between heavy chain and Protein A, homodimers can be removed by using the binding difference between Protein A and homodimers. Meanwhile, the design of different light chains can prevent mismatch of heavy chains, which enables the effective isolation by Protein L resin based on affinity difference between homodimers and antibodies on Kappa light chain.
Ion exchange chromatography: Based on the pI difference between target BsAb and homodimers, IEX resin can be used for the isolation. In one case study from one article[2], mass spectrum was used to determine the pI of target BsAb is 7.24 while the pI of homodimer was 8.04. Diamond Q(AEX) resin was used for the purification, under the selected sample loading and washing condition(pH is 7.5 and conductance is 5mS/cm), load sample capacity was 80mg/ml, positively charged homodimers then went through the resin, achieving good isolation of target molecules.
Mixed-mode chromatography: MMC mixed-mode resin possesses hydrophobic, hydrogen bond and thiophilism based on IEX interaction. Therefore, it is possible to remove homodimers from target BsAbs based on the disparity in charges on their surface. According to one case study from another article[3], resin selected for the isolation job was Diamond MMC Mustang, which performed the job under the following conditions: load sample pH 5.5, conductance 5mS/cm, load sample capacity 30mg/ml. During the washing steps, half antibody fragments and Knob-Knob homodimers can be effectively removed by optimizing washing conditions (50mM His-HCl, 63mM NaCl, pH6.5). Specifically, despite of the slightly strong binding of Knob-Knob than half antibody, the binding is still far weaker than target BsAb, which makes its removal in washing step possible.
2. Removal of low mol.wt. fragments
Being a common by-product in BsAb assembly, half antibody is the non-homodimer impurities caused by steric hindrance during the over-expression of partial heavy chains or light chains. 3/4 antibodies are impurities produced by antibody with one missing light chain as a result of disulfide bond breaking. The following part contains purification cases where affinity, mixed-mode, IEX resins are used for the isolation of low mol.wt. fragments including half antibodies and 3/4 antibodies.
Protein A affinity chromatography:
half antibody only contains one Fc region, which decreases its binding with affinity resin compared to those with two Fc regions. This leads to its earlier elution than target molecules by linear gradient pH or isocratic pH elution.
Mixed-mode chromatography: MIX-A resin possess hydrophobic interaction and hydrogen bond effect in addition to AEX function, leading to effective removal of impurities including aggregates, DNA, HCP, endotoxin and Protein A. According to the case of symmetric BsAb, Diamond MIX-A Mustang resin was used. When loading sample, set pH at 7.0, loading capacity 30mg/mL. ScFv-missing by-products could be effectively removed by optimizing washing conditions (20mM PB, 0.3M Arg-Cl, pH7.0). In addition, no target protein was eluted and SEC purity of eluted products could be increased by 8%.
Ion exchange chromatography:
based on the difference in charges carried by target BsAb and low mol.wt. antibody, isolation can be done by pH or salt linear gradient. In a testing of BsAb(pI was 7.7 ), MegaPoly BXS resin was chosen. Under the specific sample loading conditions(pH6.0, sample loading capacity 60mg/ml), Buffer A (20mM Citrate-Na, pH6.0) and Buffer B(20mM Citrate-Na, 0.3M NaCl, pH6.0) were chosen for elution. Performed linear gradient elution with 0-100% of Buffer B, a separate smaller peak was observed right after the elution peak of low mol.wt. fragments. According to data of SEC-HPLC and NR CE-SDS detection, SEC of collected products increased by 8.3% and CE-NR improved by 10.5%, indicating effective removal of fragments.
In a testing of the same BsAb, Diamond CD-S resin was chosen. Under the specific sample loading conditions(pH6.0, sample loading capacity 60mg/ml), Buffer A (20mM Citrate-Na, pH6.0) and Buffer B(20mM Citrate-Na, 0.3M NaCl, pH6.0) were chosen for elution. Performed linear gradient elution with 0-100% of Buffer B, a separate smaller peak was observed right after the elution peak of low mol.wt. fragments. According to data of SEC-HPLC and NR CE-SDS detection, SEC of collected products increased by 7.3% and CE-NR improved by 11%, indicating effectiveness in fragment removal of Diamond CD-S resin.
3. The removal of high polymer
During the production process of BsAbs, the extension of peptide chain improves length and flexibility of peptide chain, which further contributes to entanglement among molecules and therefore leads to the formation of aggregates. Besides, low pH used in affinity chromatography elution will also uplift the possibility of aggregate formation. The following example shows the isolation of high polymer by using HIC and mixed-mode chromatography approach.
Hydrophobic interaction chromatography:
HIC enjoys outstanding performance in the removal of aggregates. Since aggregates enjoy better hydrophobicity than monomers, target molecules will flow through HIC resin and therefore bind and isolate aggregates from target molecules. Specifically, HIC resins with weaker hydrophobicity can be used for target molecules with high hydrophobicity.
HIC resin with high resolution can also be used for isolation of aggregates and target antibodies. According to another published case study[4], the target product was symmetrical BsAb and resin used was Phenyl Bestaorse HP. A normal decreasing linear elution with sodium sulfate was engaged to determine the baseline purity and recovery. When using decreasing linear gradient elution with sodium sulfate (0.4-0M) at pH 8, isolation was unsatisfying according to elution peaks on chromatogram. Furthermore, recovery for collected products (SEC>99%) was merely 35.7%.
After the optimization of elution condition, which was decreasing linear gradient elution with sodium sulfate(0.4-0M)+increasing linear gradient elution with arginine (0-1M), significant tailing was observed on elution peak and recovery for collected products (SEC>99%) improved dramatically by 46% to 81.7%, indicating a successful attempt to isolate aggregate from target molecules.
Mixed-mode chromatography:
Diamond MMC Mustang enjoys wide application in BsAb purification, particularly in the clearance of byproducts including degraded fragments, charge isomers and aggregates. According to a published case study[5], target products was asymmetrical BsAb and Diamond MMC Mustang was chosen. Under certain sample loading conditions (ammonium sulfate concentration was 1M, pH 7.0, loading capacity was 30mg/mL), Buffer A (50 mM Tris-HAc, 1 M (NH4)2SO4, pH 7.0) and Buffer B (50 mM Tris-HAc, pH7.0) were used for elution.
Linear gradient elution was performed with 0-100% of Buffer B. As illustrated by Figure 3, impurities such as half antibody and high mol.wt. Accumulated at peak tail and therefore were effectively isolated from target BsAb.
To be more specific, the isolation was achieved by the disparity in hydrophobicity of target BsAb and high polymers. Meanwhile, as illustrated by the comparison chromatogram of Diamond MMC Mustang and a similar MMC resin from a reputable international brand, the two resins achieved comparable and almost consistent isolation performance and product quality.
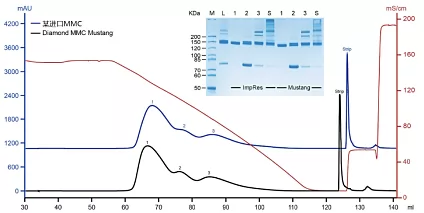
Fig.3 Chromatograms of Diamond MMC Mustang and similar MMC resin from a well-known global brand (under same elution condition)
Conclusion
The complex structure of BsAb poses new challenges for purification task. However, purity and yield of target BsAb can be effectively improved via well designed purification strategy. Various chromatography resins including affinity resin, IEX resin, Mixed-mode and HIC resin can be used based on specific application requirements and merits of each resin type.
By the effective combination of different available resins, impurities can be reduced to the lowest possible level in the polishing step, contributing to more stable downstream processing performance and finally get releasing standard-compliant BsAb products. You are welcomed to contact Bestchrom technical support team to share your valuable thoughts/experience on BsAb purification application.
Product Information
|
Resin |
Type |
Pack size |
Cat. No |
|
Diamond Q |
AEX |
25mL |
AI0111 |
|
Diamond MMC Mustang |
Mixed-mode |
25mL |
AI0161 |
|
MegaPoly BXS |
CEX |
25mL |
AI05701 |
|
Diamond CD-S |
CEX |
25mL |
AI05501 |
|
Phenyl Bestarose HP |
HIC |
25mL |
AH201105 |
Reference:
[1] Chen, Serene W, and W. Zhang. (2021)Current trends and challenges in the downstream purification of bispecific antibodies. Antibody Therapeutics
[2] Chen, Wei, et al. (2023) Resin and loading condition screening for effective and robust byproducts removal by anion exchange chromatography: A case study. Protein expression and purification
[3] Tang, Jiaqin, et al. (2020) Removal of half antibody, hole-hole homodimer and aggregates during bispecific antibody purification using MMC ImpRes mixed-mode chromatography. Protein expression and purification
[4] Hall, Troii, G. M. Kelly, and W. R. Emery. (2018) Use of mobile phase additives for the elution of bispecific and monoclonal antibodies from Phenyl Sepharose HP and Capto Phenyl ImpRes Resins. Journal of Chromatography B
[5] Zhang, Shengwei, et al. (2021) Evaluating a new mixed-mode resin Diamond MMC Mustang using Capto MMC ImpRes as a benchmark. Protein Expression and Purification








.png)


