Strategy for the key process of effective lentivirus purification
Introduction
Lentivirus, as an excellent viral vector, has been widely used in cellular immunotherapy. Particularly, its application in Car-T therapy has provided a new direction for the treatment of tumor and immune diseases.
Lentivirus, derived from human immunodeficiency virus(HIV), belongs to retrovirus categories .It achieves stable expression of exogenous genes via integration of foreign genes into the host gene sequence. Currently, by operations including membrane protein reconstruction, removal of regeneration-related and toxic genes, scientists have successfully provided lentivirus higher security and wider host infection ability, in addition to its advantages such as high viral load and stable expression of exogenous genes.
Characteristics of lentivirus
In addition to its unique advantages in gene therapy, lentivirus poses new challenges for production and purification:
● Single-strand RNA virus
● Cytolytic type
● Diameter 80-120nm
● Viral load: max 9kb
● Viral envelope structure: sensitivity to low pH, high temperature, shear force and salinity.
Production process of lentivirus
Currently, lentivirus production is mainly based on the plasmid transfection of HEK 293 cells for adherent/suspension cell culture. Steps of the process include: HEK293 cell culture, clarification, nuclease processing, ultra-filtration & concentration, chromatography purification, ultra-filtration & buffer change, as well as sterilization & filtration.
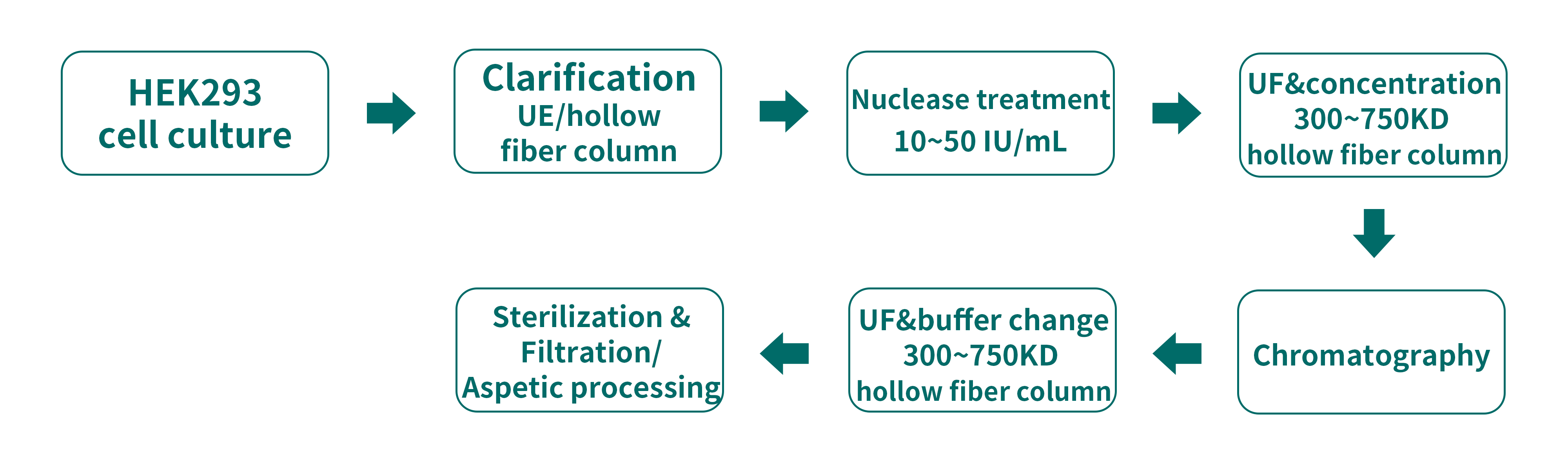
Activity of lentivirus mainly depends on its envelope structure, which has some glycoproteins being sensitive to low pH, high temperature, shear force and salinity. Any damage to viral envelope will lead to inactivation of virus. Therefore, certain conditions need to be controlled during production. For instance, 300-750KD hollow fiber column can be used in ultrafiltration(UF) to effectively reduce shear force and pressure.
Specifically, keep shear force <4000/s-1, TMP to 3~7psi, concentration ration to 5-10. During chromatography, reduce lentivirus’ exposure to high salinity and add moderate amount of stabilizer. Because of the relatively big size of lentivirus, yield of virus is usually at 60%-70% after 0.22μm sterilization filtration. Therefore, aspetic processing is usually applied to improve yield. For recovery rate in process, recovery rate of infectivity tier should be a key. QPCR or FACS methods can be used for infectivity tier detection.
Purification strategy of lentivirus
Before chromatography step, nuclease treatment and filtration steps are performed to remove a part of impurities from lentivirus. However, impurities including HCP (host cell protein) and HCD(host cell DNA) will not be removed without chromatography step. Based on the difference of impurity size and PI between the target lentivirus and impurities, mixed mode resin and AEX resin are used for further purification.
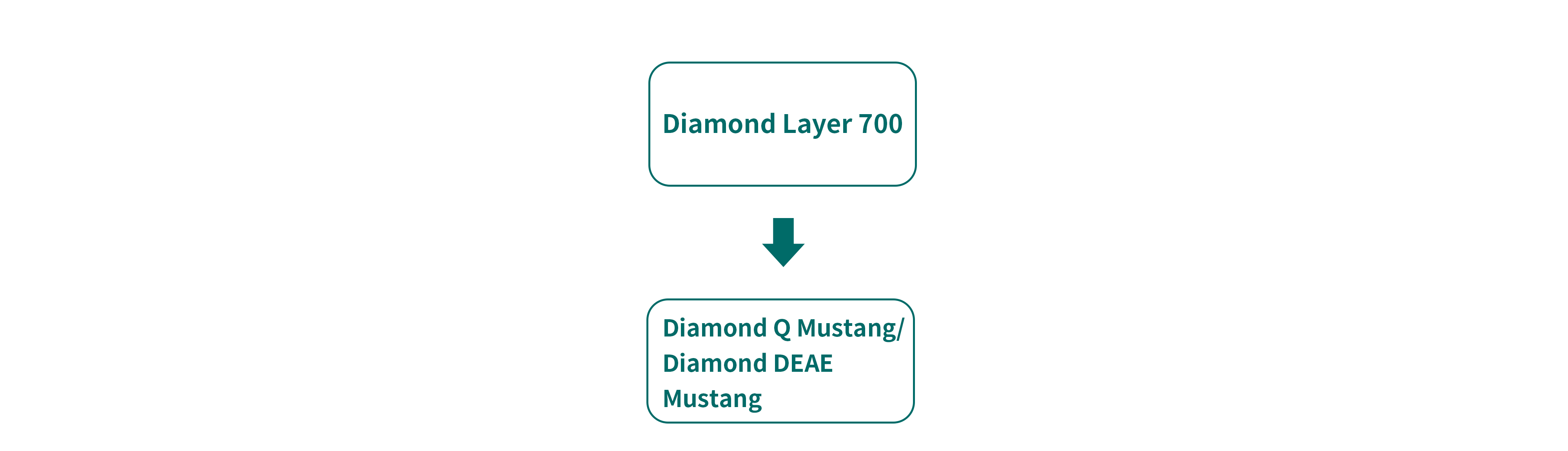
Diamond Layer 700 is a mixed mode resin designed for the purification of virus and macro-molecules. Its unique double-layered structure block the macro-molecule virus from entering into the pores and therefore directly flow through. Meanwhile, most impurities will bind on ligands inside the microspheres via ion exchange and hydrophobic interactions. The resin enjoys advantages such as high operational flexibility, high loading volume and fast flow rate, as well as higher efficiency compared with traditional 4FF/6FF resins.
● Case study: Diamond Layer 700 for lentivirus purification.
● Sample loading volume is 0.3-0.6CV, virus infection yield can reach 60% or above.
● Ability to meet quality standard by one-step purification when there is relatively a small number of upstream impurities.
Diamond Q Mustang and Diamond DEAE Mustang are AEX resins with high resolution, which can be used for the removal of impurities such as HCP, HCD and endotoxin. Diamond Q Mustang enjoys higher binding capacity than Diamond DEAE Mustang resin. However, due to its quaternary amination ligands, Diamond Q Mustang resin might have certain impact on the envelope of lentivirus and therefore result in a far lower recovery rate. In conclusion, the choice of right AEX resin will ensure product quality with optimized process condition to suit virus property.
It is noteworthy that, lentivirus is highly sensitive to high salinity, which means 500mM NaCl elution buffer shall be diluted to less than 200mM right after elution in AEX purification process to prevent any damage to viral structure. To be more specific, infective activity of virus will be reduced by 50% after exposing lentivirus to 1M NaCl for 1 hour. Meanwhile, it is recommended to add 5% cane sugar as additive to keep the activity of virus.
Conclusion
Effective purification of lentivirus is crucial for the gene therapy and cellular immunotherapy. The right choice of resin and optimized purification process significantly improve recovery rate and purity of lentivirus. Bestchrom provides appropriate options such as Diamond Layer 700 and Diamond Q/DEAE Mustang resins, which will be effective purification solutions to quality lentivirus production.








.png)


