Aggregate removal| The application of HIC resin in bsAbs purification
Introduction | Challenges and opportunities of bsAb
Bispecific antibodies(bsAbs) is artificial engineered IgG, which can recognize two epitopes on individual or several antigens. The dual targeting ability makes them more advantageous compared with traditional monoclonal antibodies(mAbs), providing bright future for drug therapeutics.
The first bsAb-based drug, catumaxomab, was launched to market in 2009. The drug targets CD3 and EpCAM, is used to treat malignant ascites. Despite the fact of its withdrawal from market in 2013, bsAb-based drugs have been developing rapidly since then. In 2023, over 200 bispecific antibody molecules are undergoing more than 300 clinical trials worldwide. Among which, about 75% of them are targeting solid tumors and 25% for hematological malignancies. By the end of 2025, a total number of 19 bsAb-based drugs have been launched to market.
In terms of structure, bsAbs can be categorized into Fc-depleted bsAbs (Non IgG-like BsAb) and Fc-containing bsAbs. The latter have symmetric and asymmetric types. Among them, asymmetric bsAbs have four different chains(two HC chains and two LC chains), which increases the possibility of mismatch and therefore produce large amount of by-products. For symmetric bsAbs, the contents of aggregates significantly increased despite of low level of by-products, which might be formed from intermolecular domain exchange caused by increase in chain length and flexibility. Therefore, efficient removal of impurities including mismatched products and aggregates becomes the key challenge in downstream purification of bsAbs.
Application of HIC in bsAb purification
Hydrophobic Interaction Chromatography(HIC) is widely used in the downstream bioprocessing due to its ability to remove aggregates from antibody efficiently.
Principle: based on the hydrophobic disparity on protein surface, separate proteins using reversible interaction between protein and hydrophobic surface of HIC resins.
Process mode: bind-elute or flowthrough mode
Bind-elute mode: Select resin with right hydrophobicity, add salt(high concentration) to sample, HIC resin binds to protein, elute with lower salt concentration, separate target protein and impurities.
Flowthrough mode: Select resin with high hydrophobicity, add no slat or salt(low salty concentration) to sample, target protein flows through while impurities bind to HIC resin.
Case: additives to improve resolution of HIC resin
The following table shows data from HIC optimization experiment of IgG4 bsAb(introduce ScFv to Fc region). Load SEC is 85.6%, HCP is 36ppm.
Table1. Purification data after HIC process optimization
|
Group
|
Load/wash
|
Elution
|
Recovery (%)
|
SEC (%)
|
HCP (ppm)
|
|
Fig A(control group)
|
0.4 M Na₂SO₄ load /0.4 M Na₂SO₄,pH 8 wash
|
Decreasing Na₂SO₄ gradient,10CV
|
35.7
|
99.6
|
5
|
|
Fig B
|
Decreasing Na₂SO₄ gradient +increasing hexanediol gradient(5% hexanediol),10CV
|
78.4
|
99.4
|
2
|
|
Fig C
|
Decreasing Na₂SO₄ gradient+increasing arginine gradient(1M arginine),10CV
|
81.7
|
>99.8
|
3
|
|
Fig D
|
After affinity, Load at pH5(No Na₂SO₄)/0.4M Na2SO4 , pH 8 wash
|
Decreasing Na₂SO₄ gradient+ Increasing hexanediol gradient(5% hexanediol),10CV
|
74.9
|
98.4
|
<5
|
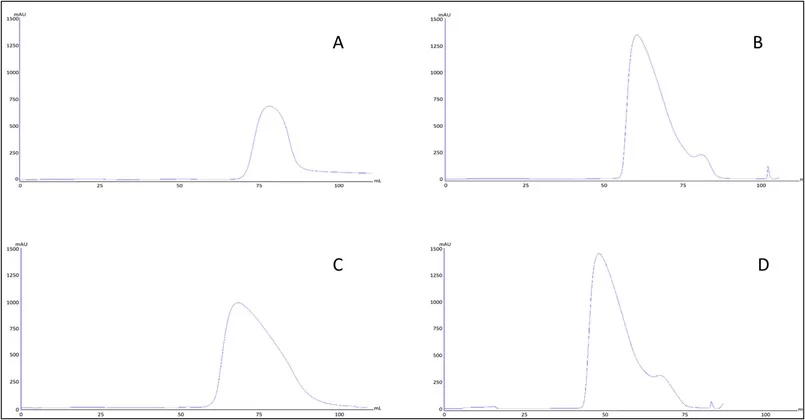
Fig.1 Impact of Hexanediol and arginine elution gradient on HIC resin separation curve
According the results:
Hexanediol and arginine can significantly improve purity and recovery of target proteins. Possible reason might be: Hexanediol and arginine can improve solution polarity and therefore decrease HIC interaction, stabilize protein conformation and optimize elution dynamics, which will eventually improve selectivity of resin. However, in practical application, the risk of additive should be considered and removed at the same time. As illustrated from this experiment, it is possible to reduce process complexity by simplifying HIC sample load condition.
The selection and optimization of HIC resins
When choosing HIC resins, it is necessary to consider factors such as hydrophobicity, ligand density and base matrix property of resins. Choose HIC resin with right hydrophobicity according to fig.2.
1、Hydrophobicity strength of HIC ligands:Butyl-S<Butyl<Octyl<Phenyl
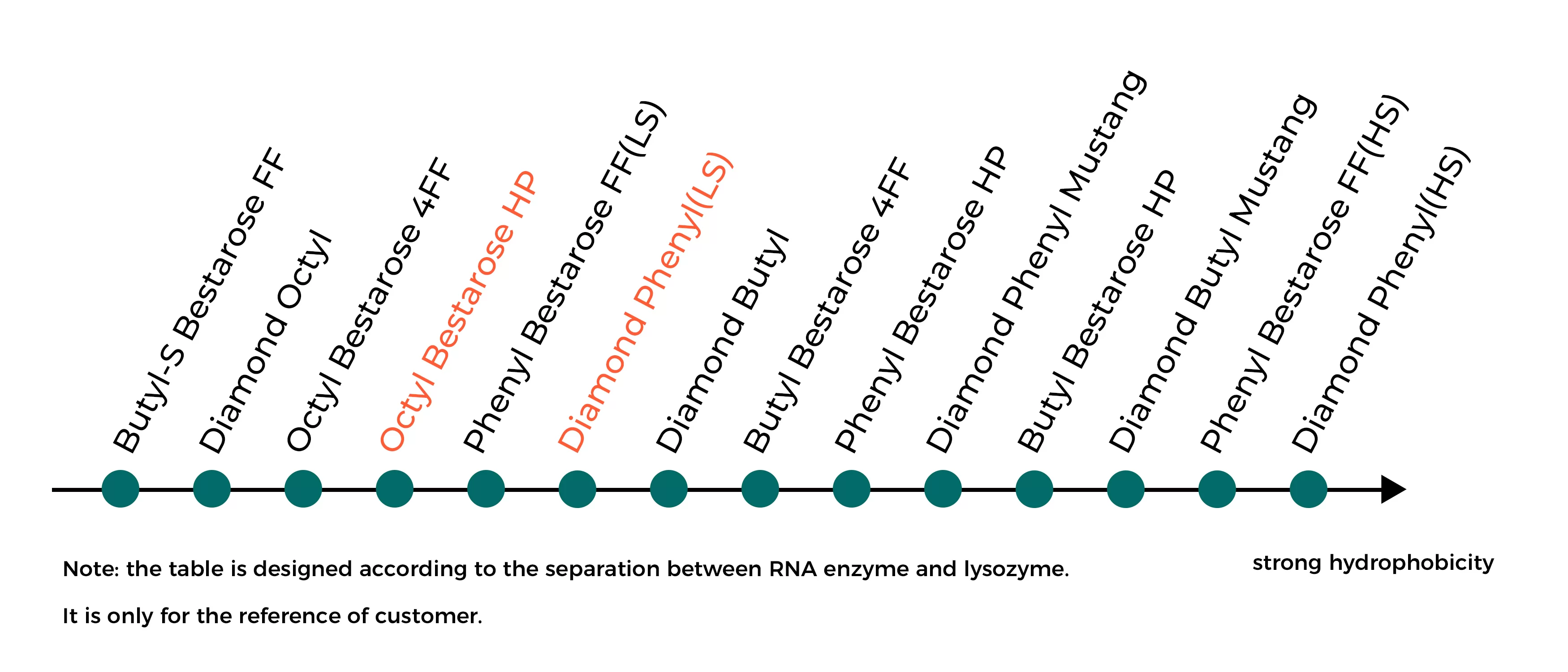
Fig.2 Hydrophobicity strength of HIC resins
2、Resolution difference based on particle sizes:HP resins(34μm)>Mustang resins(40μm)>FF resins(90μm)
3、Buffer selection: the right buffer type and concentration are significant parameters to impact binding capacity and selection of HIC resin.
The most commonly used solution is (NH4)2SO4(within 2M, pH is not above 8.0), Na2SO4 and NaCl.
See impact of different salts on HIC resin:
Na2SO4 > (NH4)2SO4 > NaCl > NH4Cl > NaBr > NaSCN
4、Selection of ionic strength: Different ionic strength has different selectivity. Since binding strength is determined by ionic strength, binding strength and protein binding capacity will increases as the improvement in salinity. However, this might cause protein precipitation due to excessive binding strength, which lead to difficulty in elution. Meanwhile, overly low salinity will lead to unbinding to protein. Therefore, the following buffer condition is recommended for the first experiment of unknown protein: binding buffer(50mM PBS pH7.0,1-1.5M(NH4)2SO4)+ elution buffer(50mM PBS pH7.0). Please adjust ionic strength based on experimental data.
5、Selection of additive: water-soluble alcohols, detergents(improve polarity of solution, reduce surface area of water and reduce interactions, leading to protein dissociation. E.g. hexanediol, isopropanol. ), dissociative salts(reduce hydrophobic interaction of solution and lead to protein dissociation. E.g. CaCl2、MgCl2 )
6、Temperature control: hydrophobic interaction is entropy-driven. As temperature rises, hydrophobic interaction and Van der Waal’s force increases, leading to stronger binding to proteins. Thus, it is necessary to control temperature in experiment.
Conclusion
HIC, as the key step in bsAb purification, shows unique advantage in the removal of aggregates. By selecting the right resin type and optimizing processing parameters, it is possible to achieve the efficient separation of target molecules from impurities, leading to high purity and recovery of target proteins. HIC resins play a key role in the scale-up of bsAb production.
Reference
[1] Klein, Christian, et al. "The present and future of bispecific antibodies for cancer therapy." Nature reviews Drug discovery 23.4 (2025): 301-319.
[2] Li, Yifeng , et al. "A roadmap for IgG-like bispecific antibody purification." Approaches to the Purification, Analysis and Characterization of Antibody-Based Therapeutics (2020).
[3] Hall, Troii. , G. M. Kelly , and W. R. Emery . "Use of mobile phase additives for the elution of bispecific and monoclonal antibodies from phenyl based hydrophobic interaction chromatography resins." Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 1096(2018):20-30.
[4] Chen, Serene W , and W. Zhang . "Current trends and challenges in the downstream purification of bispecific antibodies." Antibody Therapeutics 4.2(2021).
[5] Andrews, A. T. . "Protein purification principles, high resolution methods and applications." Food Chemistry (1991).








.png)


