Tips for Getting the Right Resin for His-tagged Protein Purification
Protein purification consists of a key step in the downstream processing of recombinant protein(Fig.1). Due to the diversity of protein structure and chemical property, tag affinity resin has become effective solution for high throughput protein purification. In addition to the specific enrichment of low abundance protein in complex condition, it can boost yields of target protein as well as bring new property to target proteins.
His-Tag(Generally are 6 Histidines) is the most commonly used affinity tag for protein purification.
-
Low molecular weight, easy expression, low immunogenicity
-
Capable of building combined tag with other types of tags
-
Mild elution condition, easy regeneration
-
Good cost-efficiency
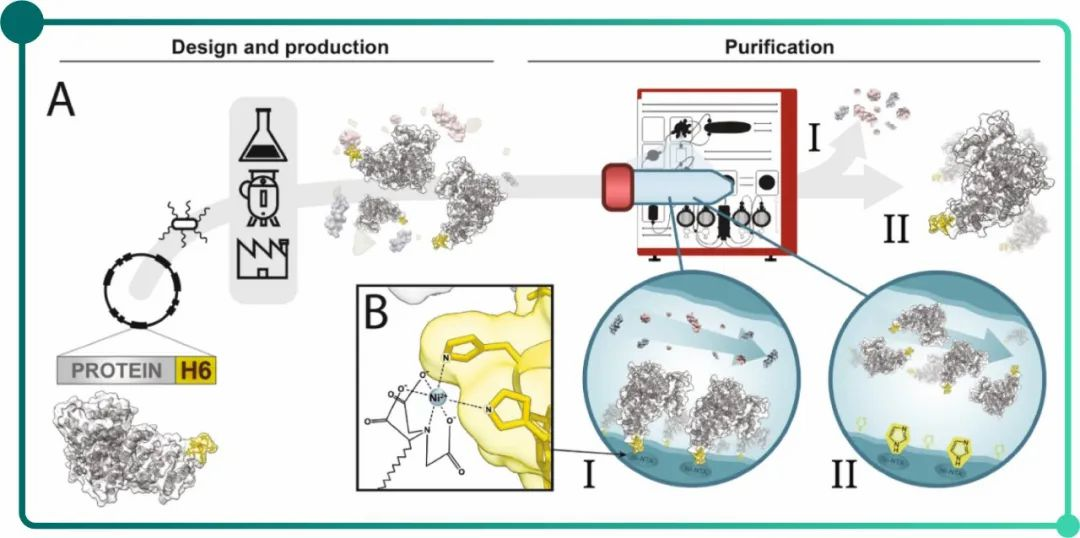
Fig.1. A. flow chart of pure recombinant peptide obtaining via target protein gene fused His Tag. B. Amplification of coordinate covalent bonds between Ni-NTA and histindine imidazole ring.
Note: The process involves gene design, cloning, cell transformation, production and Ni column (I)binding based on His-tag, before elution with imidazole(II).
Principle of His-tagged protein purification
Electron-donor groups of the imidazole ring in histidine residues can form coordination bonds with metal. Thus, purification of target proteins can be achieved by changing force between tagged proteins and metal ions.
Generally speaking, add high concentration imidazole to buffer, which can elute target proteins by competition in binding with metal ions between imidazole and histidine-tagged proteins.
Alternatively, it is also possible to achieve purification purpose by decrease buffer pH , which will weaken the binding between tagged protein and metal ions.
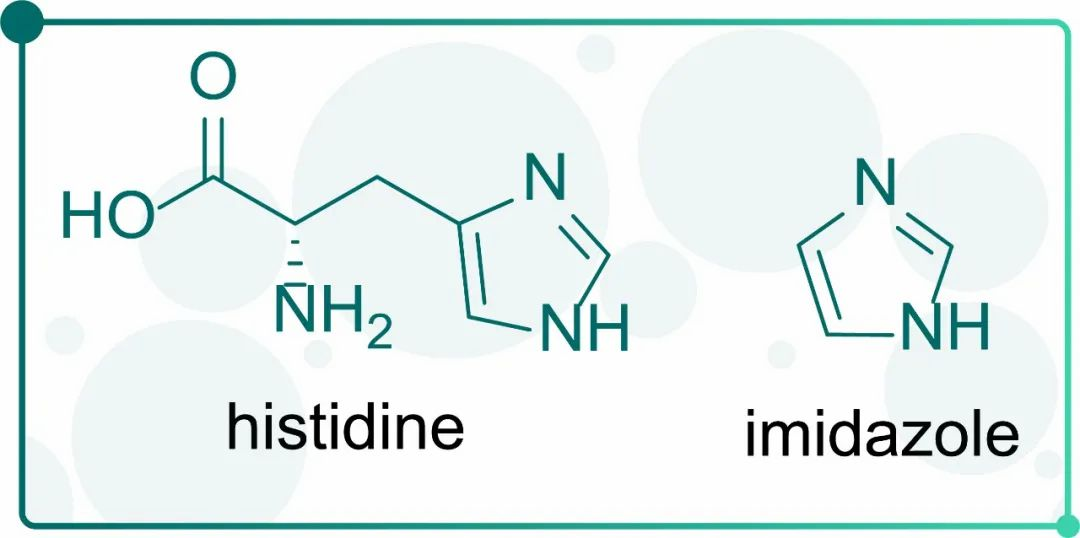
Fig.2 Molecular structure of histidine and imidazole
His-tagged protein purification solutions offered by Bestchrom
For the purification of His-tagged protein, Bestchrom offers customers with three types of His-tag purification resins, including Ni Bestarose FF/HP(pre-chelated with Ni2+) as well as IMAC Bestarose FF/HP and Chelating Bestarose FF(without pre-chelated metal ions).
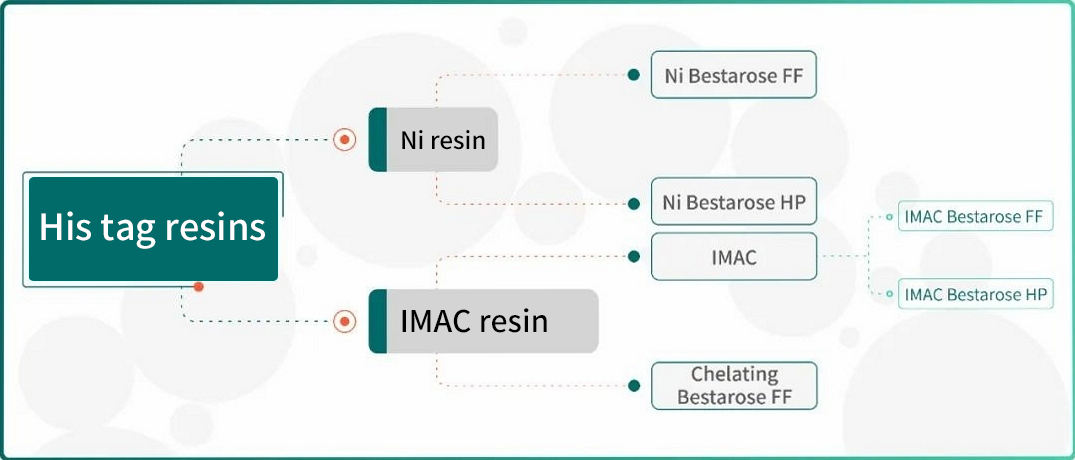
Fig.3 His tag purification resins provided by Bestchrom
Ni Bestarose FF/HP is affinity resin obtained by Ni2+ chelated nitrilotriacetic acid(NTA) ligands on 6% highly crosslinked agarose matrix.
With average particle size of 90 μm, Ni Bestarose FF enjoys advantages including high binding capacity, high flow rate and easy scale-up. The average particle size of Ni Bestarose HP is 34 μm, providing higher resolution compared with Ni Bestarose FF.
IMAC Bestarose HP/FF and Chelating Bestarose FF are resins without pre-chelation of metal ions, providing customers with options of metal ions(Co2+、Cu2+、Zn2+、Ca2+) to be coupled.
The two resins adopt different coordination methods, specifically, Chelating Bestarose FF ligands offer 3 binding sites for metal ions, while keeping 3 ionic bonding sites for affinity binding with target proteins. By contrast, IMAC Bestarose FF resin offers 4 binding sites for metal ions and 2 ionic bonding sites for target proteins.(Fig.4)
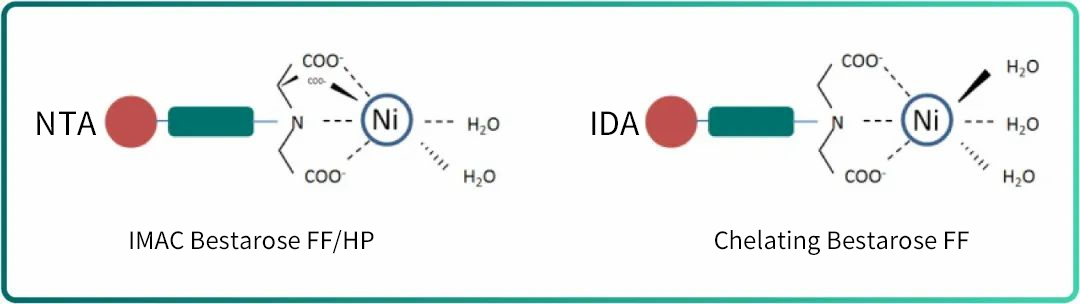
Fig.4. Coordination mode of aminotriacetic acid (NTA) and iminodiacetic acid (IDA)
In the condition of same ligand density and metal ions, Chelating Bestarose FF enjoys better affinity than IMAC Bestarose FF. Meanwhile, IMAC Bestarose FF resin has stronger binding with metal ions due to the extra chelating site, which additionally offers compatibility with reducing agent DTT and β-mercaptoethanol. Therefore, it is important to choose suitable type of resin according to protein property in the purification of recombinant histidine –tagged proteins.
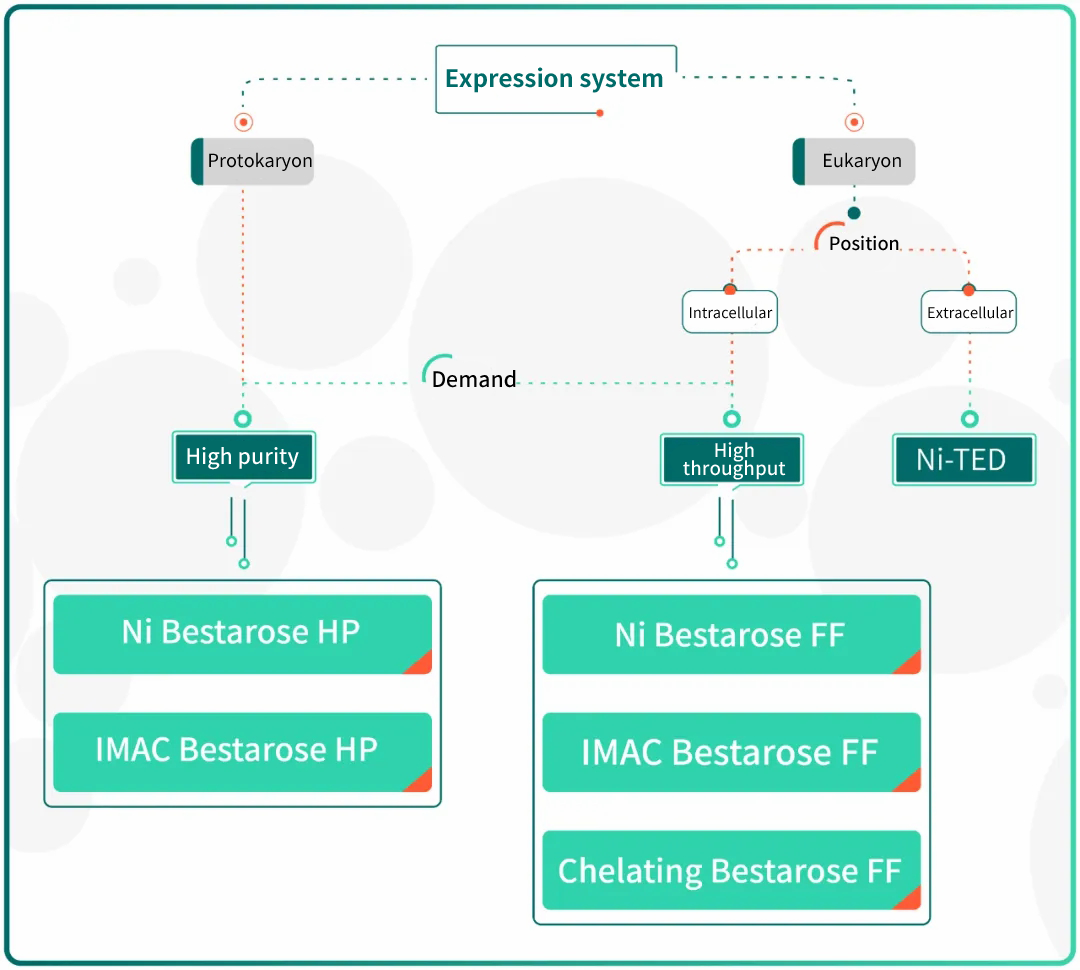
Fig.5 Guidance for His-tagged proteins purification
Application case
Application case: purification of His-tagged florescent proteins with Ni Bestarose FF resin
Resin: Ni Bestarose FF(1mL EzFast)
Sample: Lysate of E.coli
Buffer A: 25mM of imidazole pH 7.0
Buffer B: 500mM of imidazole pH 7.0
Flow rate: 1mL/min
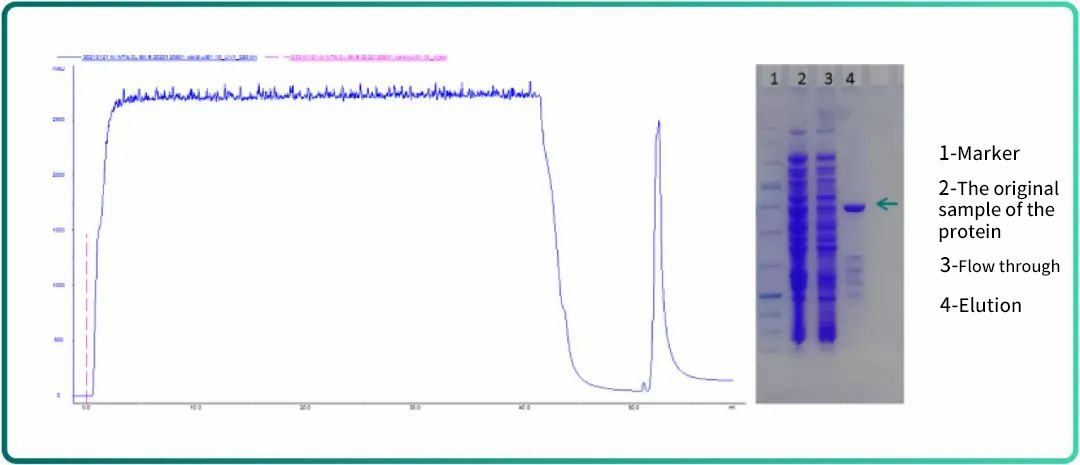
Fig.6 chromatogram and SDS-PAGE results of florescent proteins purified with Ni Bestarose FF
As illustrated by Fig.6, Ni Bestarose FF resin enjoys excellent purification performance.
Tips: Due to intolerance with strong reducing agents(DTT,EDTA) and strong chelating agents, it is necessary to exchange buffer(via dialysis or desalting) before using Ni Column for protein purification. Bestchrom’s Bestdex G-25M resin and pre-packed columns are available for the easy operation of desalting or buffer exchange. On account of the high porousness of Bestdex G-25, it is possible for rapid desalting of sample up to 30% column volume via size exclusion, dramatically boosting binding between His-tagged protein and Ni column as a result, Similarly, Bestdex G-25M can also be used in the high concentration imidazole removal of eluted sample after Ni column purification .
Technical parameters of related products

Fig.7 Resins for His-tagged protein purification
Table 1. Technical parameters of Ni resins
|
Resin |
Base matrix |
Ligand density |
DBC |
Average particle size(μm) |
Max Pressure(MPa) |
Max flow rate
(cm/h) |
pH stability |
|
Ni Bestarose FF |
Highly cross-linked agarose,6% |
12-18μmol/mL |
~40 mg His-tagged protein/mL |
90 |
0.3 |
600 |
3~12(working);2~14(CIP) |
|
Ni Bestarose HP |
Highly cross-linked agarose,6% |
~15μmol/mL |
~40 mg His-tagged protein/mL |
34 |
0.3 |
300 |
3~12(working);2~14(CIP) |
Table 2. Technical parameters of IMAC resins
|
Resin |
Functional groups |
Metal-chelating capacity |
DBC |
Average particle size(μm) |
Max pressure(MPa) |
Max flow rate
(cm/h) |
pH stability |
|
IMAC Bestarose FF |
NTA |
Ni2+/Zn2+:15μmol/mL,
Cu2+:25μmol/mL |
~40 mg His-tagged protein/mL |
90 |
0.3 |
600 |
3~12(working);2~14(CIP) |
|
IMAC Bestarose HP |
NTA |
~15μmol Ni2+/mL |
~40 mg His-tagged protein/mL |
34 |
0.3 |
300 |
3~12(working);2~14(CIP) |
|
Chelating Bestarose FF |
IDA |
~34μmol Cu2+/mL |
|
90 |
0.3 |
600 |
3~12(working);2~14(CIP) |
Table3.Technical parameters of Bestdex G-25M
|
Resin |
Matrix |
Dry particle size(μm) |
Fractionation range of linear MOL.Mr |
Fractionation range of spherical MOL.Mr |
Max flow rate(cm/h) |
pH stability |
|
Bestdex G-25 M |
dextran |
50~150μm |
100D~5kD |
1kD~5kD |
430 |
2~13 |
Reference
[1] Loughran ST, Bree RT, Walls D. Purification of Polyhistidine-Tagged Proteins. Methods Mol Biol. 2017; 1485:275-303.
[2] Waugh DS. Making the most of affinity tags. Trends Biotechnol. 2005 Jun;23(6):316-20.
[3] López-Laguna H, Voltà-Durán E, Parladé E, Villaverde A, Vázquez E, Unzueta U. Insights on the emerging biotechnology of histidine-rich peptides. Biotechnol Adv. 2022 Jan-Feb; 54:107817.
[4] Watly J, Simonovsky E, Wieczorek R, Barbosa N, Miller Y, Kozlowski H. Insight into the coordination and the binding sites of Cu (2+) by the histidyl-6-tag using experimental and computational tools. Inorg Chem. 2014 Jul 7;53(13):6675-83.








.png)


