Virus removal performance of AEX resins and influencing factors
The use of biotechnology products derived from mammalian cell lines poses a potential risk of Virus contamination. In the production process, contamination caused by endogenous or exogenous viruses is present from pre-clinical non-GMP, clinical GMP to commercialization stage, threatening the safety of patients. Statistics shows 26 cases of virus contamination of mammalian cell cultures have been reported in the past 36 years, which covered all stages of the product life cycle. Therefore, product virus safety is the key focus for both industry supervision and process development.
To ensure the safety of these products in terms of potential virus contamination, regulatory requirement of virus clearance research is an essential to evaluate the purification ability of endogenous and exogenous viruses clearance. Generally speaking, virus reduction in downstream processes is achieved by column chromatography and specific virus clearance steps (i.e. low pH incubation, detergent treatment and nanofiltration). This article will focus on virus removal effect by anion exchange chromatography (AEX) as well as influencing factors.
Virus removal mechanism and ability of AEX resins
During downstream processing, chromatography stage also serves as a key unit for virus clearance in addition to removal of process and product related impurities. Among them, AEX has been proved to be the key step in the removal of viruses.
Virus removal mechanism
When AEX is used in antibody purification projects, two modes are usually adopted:
• Flow Through Mode (FT): this mode uses the disparity of pI of target monoclonal antibody and mode viruses. Most monoclonal antibodies have high pI, which makes them positively charged and flow through under the right buffer condition (pH 7.0~8.5,low conductance). By contrast, model viruses(e.g. X-MuLV pI~5.8, MVM pI~6.2) are negatively charged under the same condition, which means they can bind positively charged resin ligands and finally be removed.
• Bind and Elute Mode (B/E): This mode uses the binding strength between monoclonal antibodies and viruses for purification. Under specific conditions, resin can bind both monoclonal antibodies and viruses, which enables selectively elute monoclonal antibodies while keeping viruses on the resin.
Virus removal capability of AEX resins
Large amount of data shows FT mode(AEX resin) can effectively remove enveloped viruses and non-enveloped viruses(see Fig.1).
• About 70% of AEX applications use FT mode while about 30% use B/E mode.
• FT mode enjoys high stability and low batch-to-batch variability in both viruses.
• B/E mode shows higher batch-to-batch variability in the removal of two types of viruses.
• The batch-to-batch variability of parvovirus is greater than that of retrovirus under both modes.
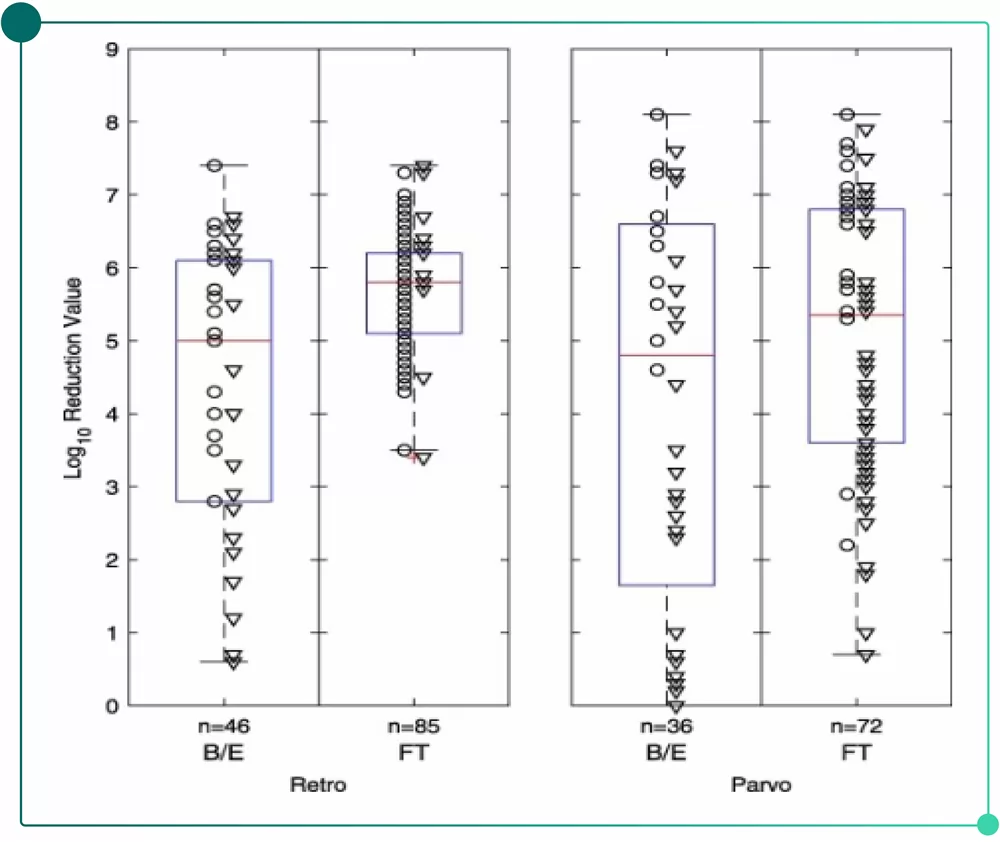
Fig.1 Data of AEX resins in the removal of retroviruses and parvoviruses(FDA database).
Virus removal ability of Mixed-mode AEX resins
Mixed-mode resins have both IEX and HIC interactions, providing unique advantages:
Increase the compatibility of buffers: enables effective operation under wider Ph and conductance range.
Better stability in virus removal: As it is showed in Fig.2, Mixed-mode AEX resins enjoy excellent removal ability for parvovirus under high salinity(High LRV under high salinity) compared with single-mode AEX resins.
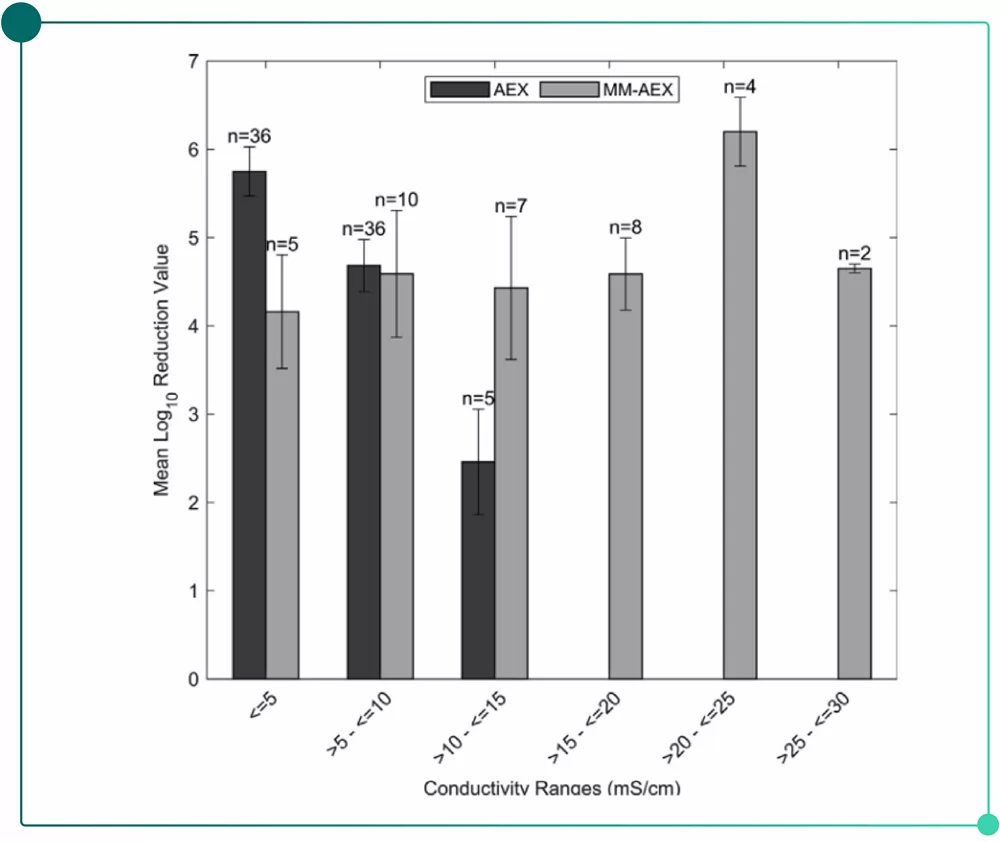
Fig.2 Parvovirus removal: Mixed-mode AEX resins VS Single-mode AEX resins(FDA database)
Interaction between Virus and resin can be influenced by factors including pI, solution pH, solution salinity, Mab property, binding sites number of virus and the existence of competing components.
• pH:The interaction between virus and AEX is static electricity. However, X-MuLV removal effect under different pH range is very stable. Interaction between X-MuLV and AEX resins will only slightly rely on pH(5.0~8.0).Different from X-MuLV, LRV of MVM at different pH(pI 6.2) is within expectation. When pH of 50mM NaCl increases from5 to 8, LRV will increase by about 4 units.
• Ionic strength:For single-mode AEX resins, virus removal performance compromises as salinity increases. By contrast, Mixed-mode resin will hardly be affected by salinity(see Fig.2).
• Mab property: For any chromatography analysis, LRV of specific viruses is impacted by both chromatography condition and mAb itself. Specifically, viruses can interact with positively charged mAb via negative charges on virus surface, which can eventually virus removal effect.
• Virus types:XpI of X-MuLV is 5.8, which is believed abnormal(X-MuLV bind AEX resins at pH 5), indicating the existence of negatively charged patches on virus surface. This therefore shows that pI is not enough to predict the interaction between viruses in complex structure and IEX resins. By contrast, when determining interaction between viruses and IEX resins, charge distribution on virus surface will be more relevant than pI.
In conclusion, AEX’s ability to remove viruses will be influenced by multiple factors. Therefore, to get stable performance, it is recommended to keep pH, conductivity and binding capacity at 7.0~8.5, <10 mS/cm and <100 mg/ml respectively when using single-mode AEX resins.
Virus removal data of Bestchrom AEX resins
Mixed-mode AEX resin Diamond MIX-A
• Efficient removal:In one project, Diamond MIX-A shows excellent performancein the removal of enveloped and non-enveloped viruses(X-Mulv, MVM, PRV, Reo3)with a LRV of 4.5-5.34 log.
• Excellent alkaline tolerance:Under challenging high salinity(When loading sample, conductivity 22 mS/cm, pH 6.43, binding capacity ~60 mg/mL ), residence time is 5min. Diamond MIX-A shows excellent removal performance to X-MuLV with LRV reaching 3.04 log and 3.25 log respectively. This proves Diamond MIX-A’s stability under high salinity condition.
Strong AEX resin Diamond Q
The following table shows Diamond Q resin’s virus removal data in Mab projects(X-MuLV and MVM):
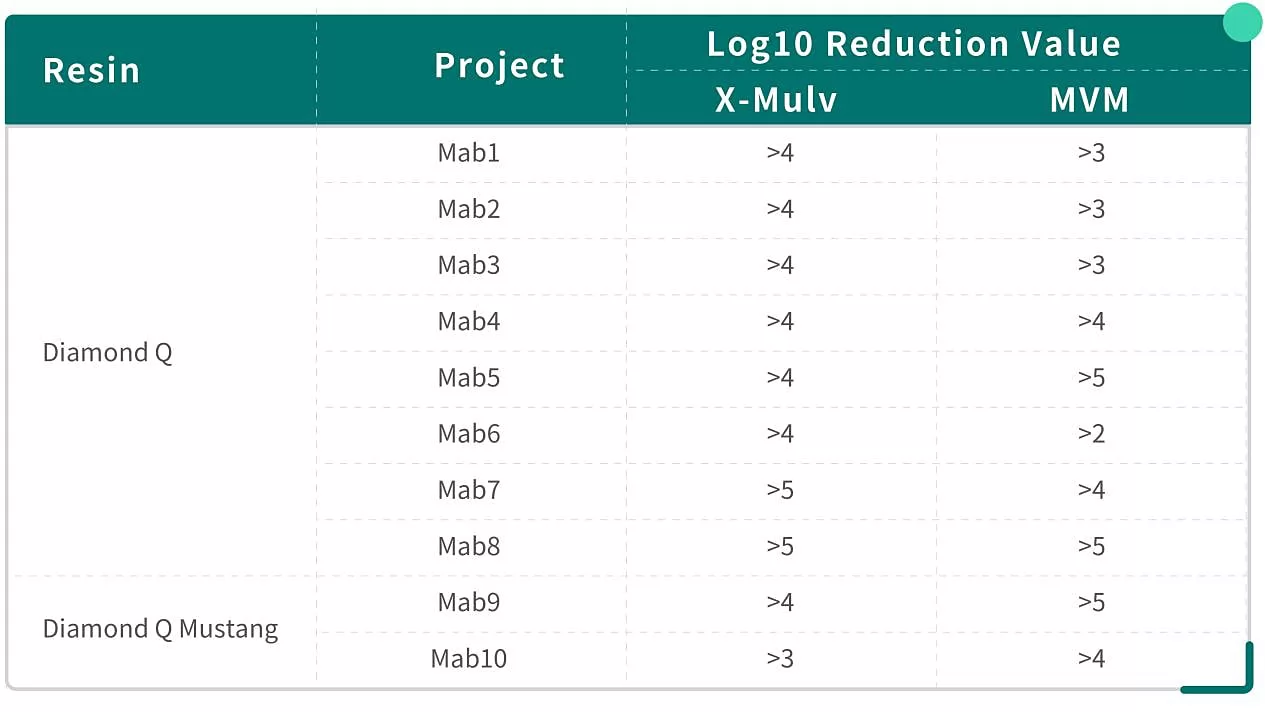
Diamond Q resin shows highly consistent virus removal ability towards retrovirus X-MuLV(all projects LRV≥4 log).
For parvovirus MVM, removal ability shows inconsistency among different projects(LRV ranges from 2 to 5 log), which confirms what was mentioned earlier. Nevertheless, LRV can still be higher than 3log in most projects.
Diamond Q Mustang resin shows comparable excellent performance in the removal of viruses in the projects.
Conclusion
Individual steps in downstream processing show different performance and stability in the removal of viruses. AEX resins are widely acknowledged as the key operation unit in the virus removal, especially under FT mode.
However, Despite of AEX resin’s reliability, its removal performance (especially towards parvovirus ) can vary based on different Mab specificity and process parameters. Mixed-mode AAE reins(e.g. Diamond MIX-A) enjoys more stable virus clearance performance under high salinity condition due to their multiple interactions.
To make sure stability of virus clearance, it is essential to operate AEX within validated process parameters range. In the virus removal study, mode viruses with representativeness and the worst process condition should be applied, so as to ensure the regulatory-compliant virus removal ability under all scenarios with potential risks.
Reference
[1] Barone, P.W., et al. Viral contamination in biologic manufacture and implications for emerging therapies. Nat Biotechnol 38, 563–572 (2020). (Updates the scope and context of viral risk).
[2] Ajayi, O.O., et al. An updated analysis of viral clearance unit operations for biotechnology manufacturing. Curr Res Biotechnol 4, 190-202 (2022). (Provides a modern overview of unit operations, including AEX).
[3] Cai, K., et al. Virus removal robustness of ion exchange chromatography. Biologicals 60, 64-70 (2019). (Specifically addresses robustness factors for IEX virus clearance, critical for the discussion on variability).
[4] Li, Y. Viral removal by column chromatography in downstream processing of monoclonal antibodies. Protein Expr Purif 198, 106131 (2022). (Focuses on chromatography for mAbs, directly supporting the application context).








.png)


